Chemistry Notes Form 1
Chemistry
Form 1 Guide
1.0.0 Introduction to Chemistry
Welcome to Chemistry, the Science concerned with the study of matter.
In simplest terms, chemistry is the science of matter.
Anything that can be touched, tasted, smelled, seen or felt is made of chemicals.
Different thing feel, taste, smell and appear differently- like the fresh vegetables.
If you are blindfolded and asked to identify potatoes, onions, carrots or cabbages from a pack, chances are you could, chances are you could- based on interactions with them in your home kitchen.
Chemistry will supply you with the knowledge and understanding to engage as informed citizens with science based issues.
You will use contemporary and relevant contexts of interest such as environmental issues to gain greater scientific knowledge.
You will study the patterns and properties of the elements and how these combine to produce useful materials, such as air and water among others.
Throughout the course you will develop practical skills, powers of analysis and the ability to apply chemical concepts to unfamiliar situations.
Being a Good Chemistry Student
You are beginning the study of one of the most interesting subjects that you will ever come across.
As is the case with all subjects, what you will get out of Chemistry will depend upon what you put into it.
Chemistry can intrigue and enlighten you.
It all depends upon the effort you are willing to put into your studies.
If you keep an open mind, and listen to your teacher, a course in Chemistry will change the way you view the world! Chemistry is a skill based course, and many of the most important lessons will come in the first few weeks.
You must start off with good study habits from the very beginning.
Below are some tips to help ensure that you get the most out of the course.
Do the reading faithfully.
Don’t try to get through the course by just listening to your teacher. Your teacher will assume that you are coming to class having read the text, and he or she will expect you to have that background knowledge required to follow the lessons.
Most importantly, you will not be able to ask clarifying questions, if you have never read the book.
Ask as many questions as you want to
Like any teacher, your Chemistry teacher uses the feedback from the class to determine whether or not his or her lessons are clear enough. The types of questions that you and your classmates ask will tell your teacher more than the results of any exam.
Some students are embarrassed to admit that something is unclear, but this subject is new to you.
There is no shame in admitting that you are not sure about a subject is new to you!
Get help early and often.
As soon as you notice that you are not following along with the class discussion you should address the problem.
There are so many sources for extra help, and you should take advantage of them.
Make an appointment to see your teacher after school, or ask one of your classmates for help.
Search the Internet for sites with additional information.
Don’t stop getting extra help until you feel confident in your grasp of the subject.
Take advantage of the learning opportunities in Laboratory classes.
Many of the lab activities that you will do will seem exciting. Violent chemical reactions will cause every heart to beat a little faster.
However, if you don’t understand the concepts behind the activities than the activities become nothing more than a magic show! Strive to understand every aspect of these activities and you will find that they are a fun way to learn Chemistry.
Do your written homework correctly.
It may seem that you are saving time when you copy homework from a friend or from the back of a textbook, but you are really causing yourself some trouble.
Each assignment is designed to reinforce a specific concept. If you don’t do the homework correctly, you may not master the concepts.
Remember that your task is never to complete a specific set of problems, but rather to master a topic or skill.
Study in groups.
Chemistry is much harder when you try to learn it in isolation. Form study groups from the first week of school.
Having people to talk to about Chemistry will help you avoid the frustration that comes from feeling you are the only one having difficulty with a particular concept or type of Math problem.
You will also be more likely to ask questions when you see that an idea may not be clear to others as well.
Some Careers in Chemistry
Chemists are the people who transform the everyday materials around us into amazing things.
Some chemists work on cures for cancer while others monitor the ozone protecting us from the sun.
Still others discover new materials to make our homes warmer in the winter, or new textiles to be used in the latest fashions.
The knowledge gained through the study of chemistry opens many career pathways. Here are just a few of the careers chosen by chemists.
• Agricultural Chemistry
• Biochemistry
• Chemical Education
• Chemical Engineering
• Consumer Product Chemistry
• Environmental Chemistry
• Food and Flavor Chemistry
• Forensic Chemistry
• Medicinal Chemistry
Some Career Descriptions:
1. Biochemistry
Biochemistry is the study of the structure, composition, and chemical reactions of substances in living systems.
Biochemistry emerged as a separate discipline when scientists combined biology with organic, inorganic, or physical chemistry and began to study such topics as how living things obtain energy from food, the chemical basis of heredity, and what fundamental changes occur in disease.
Biochemistry is applied to medicine, dentistry, veterinary medicine and food science.
2. Chemical Engineering
Chemical engineers apply the principles of chemistry, math, and physics to the design and operation of large-scale chemical manufacturing processes.
They translate processes developed in the lab into practical applications for the production of products such as plastics, medicines, detergents, and fuels; design plants to maximize productivity and minimize costs; and evaluate plant operations for performance and product quality.
Chemical engineers are employed by almost all companies in the chemical process industry.
3. Forensic Chemists
A forensic chemist is a professional chemist who analyzes evidence that is brought in from crime scenes and reaches a conclusion based on tests run on that piece of evidence.
A forensic chemist’s job is to identify and characterize the evidence as part of the larger process of solving a crime. Forensic chemists rarely conduct any investigative work; they handle the evidence collected from the crime scene.
4. Medicinal Chemistry
Medicinal chemistry is the application of chemical research techniques to the synthesis of pharmaceuticals.
During the early stages of medicinal chemistry development, scientists were primarily concerned with the isolation of medicinal agents found in plants.
Today, scientists in this field are also equally concerned with the creation of new synthetic drug compounds. Medicinal chemistry is almost always geared toward drug discovery and development.
5. Food and Flavor Chemists
Food chemistry focuses on the chemistry of foods, their deterioration, and the principles underlying the improvement of foods for consumers.
It applies chemistry to developing, processing, packaging, preserving, storing, and distributing foods and beverages to obtain safe, economical, and aesthetically pleasing food supplies.
Few people recognize the science behind the food they consume. While food science involves chemistry, biology, physics, biochemistry, microbiology, nutrition, and engineering, the major portion of a food science curriculum is chemistry.
Food chemistry encompasses everything from agricultural raw materials to consumer end-use products.
General Chemistry Laboratory
safety rules and regulations
Your school science laboratory is set up so that you can perform science experiments in safety provided that you follow the proper procedures and safety precautions listed below.
Your teacher will give you specific information about the safety routines used in your school.
It is essential for all concerned that certain rules be followed while in the lab. Read the following carefully and ask questions necessary for clarity.
1. Goggles will be worn at all times. No exceptions. Failure to wear goggles will result in expulsion from laboratory.
2. Full shoes are required. No sandal, flip-flops, etc. are allowed.
3. Lab apron is required when wearing shorts, tank tops, etc.
4. Keep locker drawers closed when not in use.
5. Do not leave flames unattended. Turn burners off when not in use.
6. Remember that most chemicals are flammable, toxic, carcinogenic or all three. Treat them accordingly. Do not ingest chemicals.
7. Acquaint yourself with the eyewash station, safety shower and fire-fighting equipment. You are responsible for knowing their location and use.
8. No smoking, chewing, eating or drinking allowed in the laboratory.
If you are taking a prescription or other drug that will affect your alertness, notify your instructor before going into lab.
9. No students are allowed in the stockroom. No lab visitors without permission of the lab instructor.
10. Report all accidents or injuries to the instructor immediately!
11. If you do not understand a procedure or you cannot read a label, contact the instructor.
Do not gamble with your (and others) safety when there is a question. What you don t know can hurt you. Ditch the foolish notion that asking questions will make you look stupid.
Some Further Explanations
1. Do not pipette by mouth
You say, “But it’s only water.” Even if it is, how clean do you think that glassware really is? Using disposable pipettes? I know lots of people who rinse them and put them back! Learn to use the pipette bulb or automated pipetter.
A Material Safety Data Sheet (MSDS) should be available for every chemical you use in lab.
Read these and follow the recommendations for safe use and disposal of the material.
2. Dress appropriately (for chemistry lab, not fashion or the weather)
No sandals, no clothes you love more than life, no contact lenses, and long pants are preferable to shorts or short skirts.
Tie long hair back. Wear safety goggles and a lab coat.
Even if you aren’t clumsy, someone else in the lab probably is.
If you take even a few chemistry courses you will probably see people set themselves on fire, spill acid on themselves, others, or notes, splash themselves in the eye, etc.
Don’t be the bad example to others, remembered for all time for something stupid!
3. Identify the Safety Equipment
And know how to use it! Given that some people (possibly you) will need them, know the locations of the fire blanket, extinguishers, eyewash, and shower.
Ask for demonstrations! If the eyewash hasn’t been used in a while the discoloration of the water is usually sufficient to inspire use of safety glasses.
4. Don’t Taste or Sniff Chemicals
For many chemicals, if you can smell them then you are exposing yourself to a dose that can harm you! If the safety information says that a chemical should only be used inside a fume hood, then don’t use it anywhere else. This isn’t cooking class – don’t taste your experiments!
5. Don’t casually dispose of chemicals down the drain
Some chemicals can be washed down the drain, while others require a different method of disposal.
If a chemical can go in the sink, be sure to wash it away rather than risk an unexpected reaction between chemical ‘leftovers’ later.
6. Don’t eat or drink in lab
It’s tempting, but dangerous… just don’t do it.
7. Don’t play mad scientist
Don’t haphazardly mix chemicals! Pay attention to the order in which chemicals are to be added to each other and do not deviate from the instructions.
Even chemicals that mix to produce seemingly safe products should be handled carefully.
For example, hydrochloric acid and sodium hydroxide will give you salt water, but the reaction could break your glassware or splash the reactants onto you if you aren’t careful.
8. Take data during lab
Put data directly in your lab book rather than transcribing from another source (e.g., notebook or lab partner).
Not after lab, on the assumption that it will be neater.
There are lots of reasons for this, but the practical one is that it is much harder for the data to get lost in your lab book. For some experiments, it may be helpful to take data before lab.
Student Activity
Crash, bang , wallop! Here is a disaster zone! But a real school chemistry laboratory is one of the safest places in which to work. Good chemists always work carefully and safely.
The rules of the laboratory have been forgotten by these students.
1. Look carefully at the disaster zone above.
a. Make a list of as many of the dangers as you can
b. From this list, make a set of Rules of the Laboratory
2. Draw a rough sketch of the laboratory you normally use. Mark on it where the following items are usually kept.
a) Bunsen burners
b) Clamps and retort stands
c) Beakers
d) Test tube racks
3. Very often, safety rules in the laboratory are written negatively and start with don’t do this, Don’t do that, you should NOT ……………………..
Rewrite your Rules of the laboratory positively by starting with Do this, Always, You should …..
The scientific method
The scientific method is a set of ideas or a procedure that scientists use to investigate things they want to understand. By using the method, you can be sure you’re carrying out your project correctly.
The scientific method allows you to investigate an experiment in a step-by-step method.
Problem: What are you going to solve in the lab? The problem or purpose explains exactly what you hope to accomplish in the investigation.
Hypothesis: How do you think it is going to turn out? Use the facts you already know to come up with a guess that might really make sense.
Materials and Apparatus: List what equipment you will need to complete the experiment. (Include diagrams of set up apparatus if required to do so in this section.
Procedure: What you must do to complete the experiment. Write down the steps you need to follow.
Data and Work: Include the tables, observations and work you did during the experiment.
This section is where you keep very careful notes on everything you do and everything you find out.
Be sure you write down or draw what really happened, even if it’s not what you thought would happen.
At the end, you look over all your data and think about it very hard.
You think of the results of your procedure, or how everything turned out. Analysis Questions: You do not need to write out questions, but you must answer in sentences that include the question.
Conclusion: You must say what you found out during the lab. You figure out whether your results agreed with your hypothesis or not. Put everything you observed together and try to make some sense out of it.
**Hint** The conclusion should answer the problem.
Remember!
1. Be neat. Your lab should be organized and easily readable.
2. Hand in a good copy. You can take a rough copy of data tables into the lab and then recopy for your hand-in report.
3. Hand in lab reports on time!
Learning activity
1. Name two basic storage and chemical handling apparatus.
2. Name one accurate apparatus for measuring liquid volume.
3. How do you determine the mass of an object?
4. Name two safety devises to prevent chemical splash.
5. Very often, safety rules in the laboratory are written negatively and start with ‘don’t do this, don’t do that, you should not……..
Rewrite your Rules of the Laboratory positively by starting with ‘Do this, Always, You should……….
Names of Common Laboratory Apparatus
Which apparatus?
You wouldn’t dream of trying a chicken using a spoon, or of drying your hair in over a stove! In everyday life, we need to use the right tools for the job we are doing.
The same is true in the science lab.
There are lots of types of different chemical apparatus, all designed to do different jobs.
Using the right apparatus makes your practical work safer.
The pictures below show some of the apparatus you will be expected to know about and use in a chemistry laboratory.
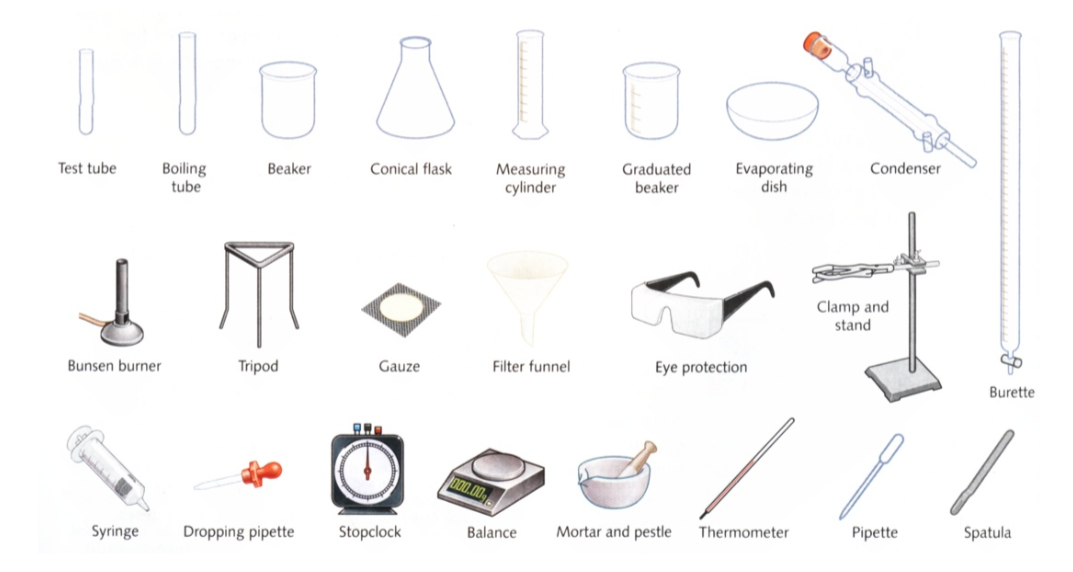
Burette
Made from accurate uniform wall tubing to insure the stipulated capacity tolerances. Features durable, permanent markings; fine, sharp lines and large, easy-to-read numbers.
The stopcock is carefully ground and finished to assure a leak-free operation.
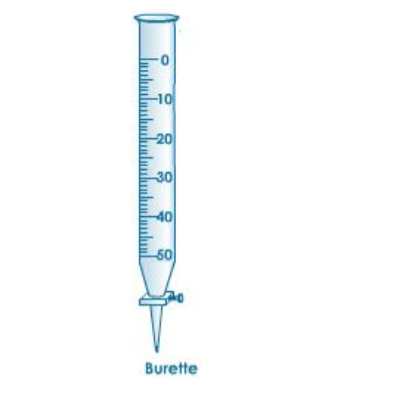
A burette is used to deliver solution in precisely measured, variable volumes.
To fill a burette, close the stopcock at the bottom and use a funnel.
You may need to lift up on the funnel slightly, to allow the solution to flow in freely.
Pippette
A pipette is used to measure small fixed amounts of solution very accurately. A pipette bulb is used to draw solution into the pipette.
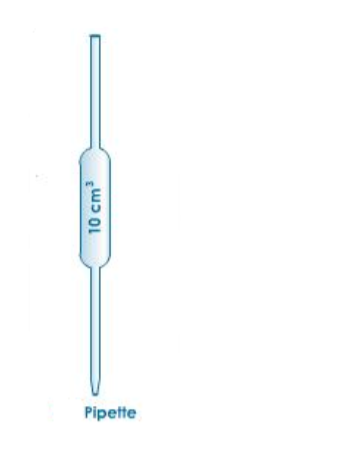
A pipette has a mark that shows how much volume it can draw. It cannot be used for transferring any other volume unless the one specified on it.
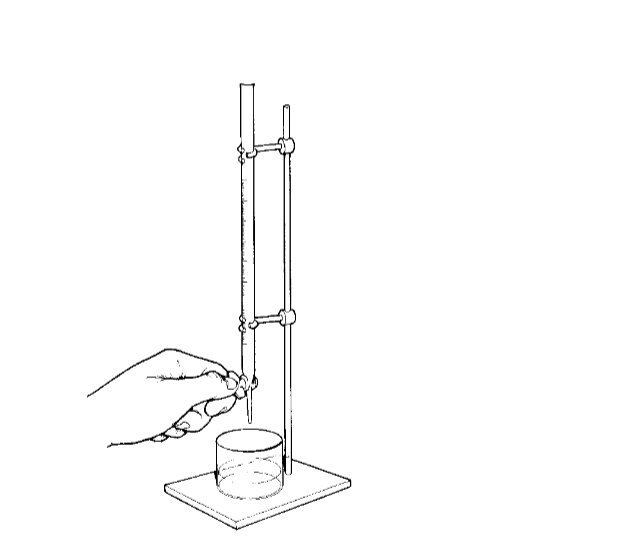
Using a Pipette
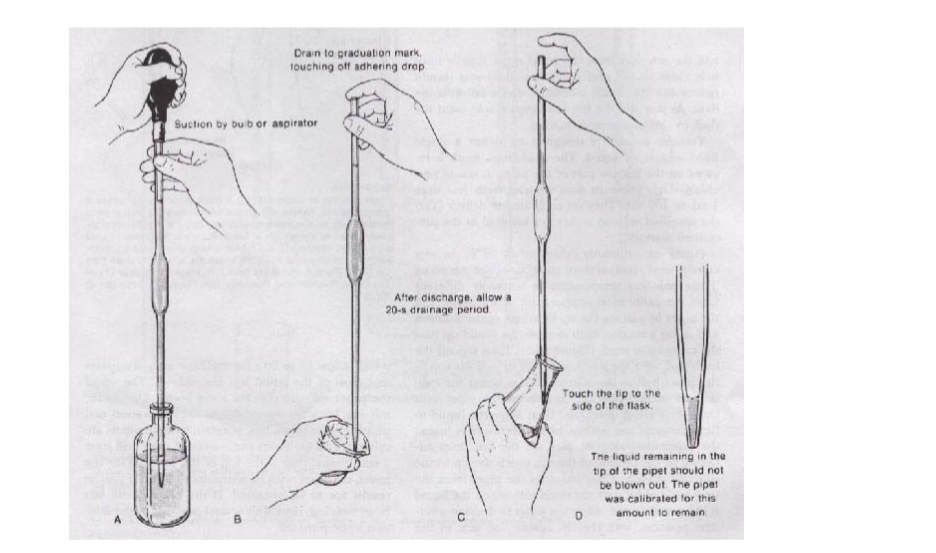
3. Beakers
Made of heat resistant borosilicate glass, withstanding temperatures up to 680°C, the beaker features a large marking spot, graduations and pouring spout.
The primary function of a beaker is to hold and work with liquids.
If graduated, it can serve to make approximate measurements of liquid volume.
The beaker is made of specialized glass so it can be heated and cooled without breaking. This type of glass makes the beaker brittle and it must be handled carefully.

Evaporating Dish

This porcelain item resembles a shallow bowl with a spout. Evaporating dishes are traditionally used to evaporate solvent to concentrate a solution; however they can also be used to hold sand for a sand bath, as a small water bath, or as a drying dish (like a watch glass).
Brushes

Glass rods
These rods are used in all science laboratories to stir and mix substances. 5mm in diameter and 200 mm (approx. 8 inches) in length.
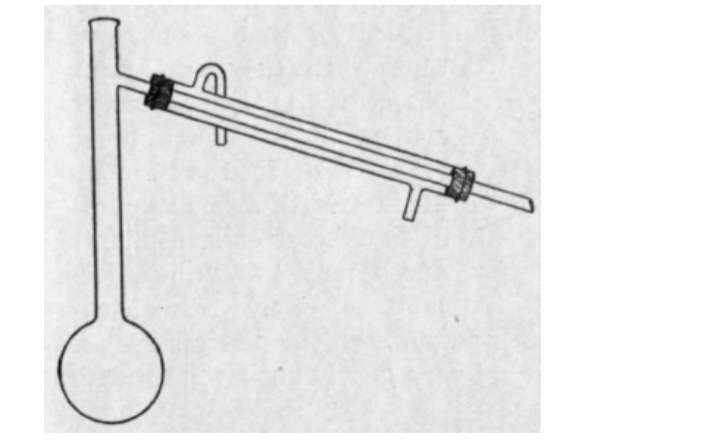
Condenser
This apparatus is made of high quality borosilicate glass. It can withstand up to 600°C temperature.
This instrument is suitable for pure water distillation and other kinds of distillation in the laboratory.
The Graham condenser has both inner and outer joints. For use in vacuum distillation, the jacket is sealed to the inner tube.
Drip tip is located at the end. Hose connections are 10mm overall diameter. Top opening is 29mm. Cold water enters from the lower tap and exits from the top.
4. Googles.
Eye protection is a priority in any science laboratory setting. When fumes are involved (and you will be working with A LOT of those in chemistry) and well as possible splashing, you generally need something that does not allow air to vent through.
Protective goggles that are meant specifically for chemistry have plugs to keep those fumes/liquids out.
It’s also why you have eyewash stations in your lab in case someone does not wear their goggles and need to wash the chemicals out of their eyes.

5. Stirrer
The function of a stirrer is to agitate liquids for speeding up reactions or improving mixtures.

6. Measuring Cylinder
Graduated or measuring cylinders are specifically designed to make accurate liquid volume measurements.

The volume is read from the lowest portion of the meniscus of the liquid; that is, the lowest portion of the convex dip of the liquid as it sits in the graduated cylinder. Graduated cylinders are available in a number of sizes.
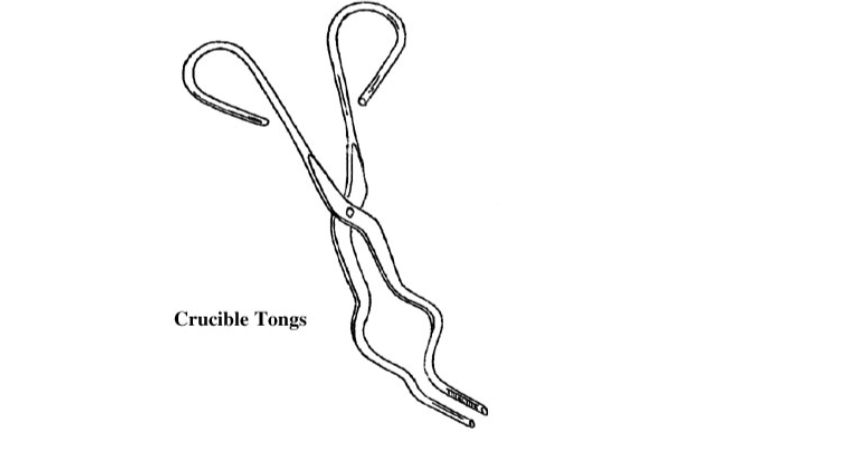
7. Crucible Tongs
Used for handling or manipulating hot crucibles and similar apparatus.
They have corrugated tips for easy handling; riveted joints and the finger openings are large enough for a firm grip. They are made of plated steel.
7. Mortar and Pestle

Mortar and pestles are used to grind solids into powders.
8. Test Tube
The function of a test tube is to hold a small experiment, which would be used to conduct an investigation.
The test tube is made of specialized glass so it can be heated and cooled without breaking. This same type of glass makes the test tube brittle and it must be handled carefully.
Test-tube Rack
9. Thermometer
The thermometer is an instrument for determining temperature.
Most laboratory thermometers are calibrated in the SI scale (degrees Celsius).

10. Thistle Tube
Thistle tubes are usually used in experiments involving semi-permeable membranes. Osmosis and diffusion can be illustrated well.

Thistle tubes are also used in separation experiments as well as other chemical applications.
11. Balance
The function of a balance is to mass objects.
[NOTE: If the device is measuring an object using springs against gravity, the devise is correctly referred to as a scale and its measurements are described as weight not mass.]
A balance uses a comparison of a known substance or calibration with the unknown object to determine the unknown object’s mass.

12. Fume Hood
Fume hoods protect laboratory workers from fumes and potentially dangerous chemical reactions by continuously vacuuming air out of the lab and by providing a glass shield.
Experiments can be clearly seen by the user, yet the user is protected from splatter and harmful fumes.
13. Funnel
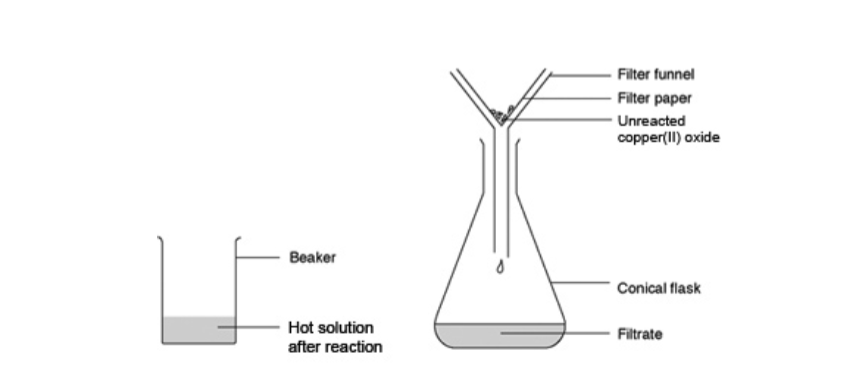
A funnel is used to aid in the transfer liquid from one vessel to another.
14. Dropper
A dropper is used to transfer a small volume of liquid (less than one mL).

15. Volumetric Flask
A volumetric flask is used to measure very precisely one specific volume of liquid (100 ml, 250 ml, etc., depending on which flask you use).
This flask is used to prepare a solution of known concentration.
A volumetric flask should not be used to heat substances or store solutions, and you should avoid pipetting directly from the volumetric flask.
Our volumetric flasks provide precise volume measurement.
The necks are tooled and have glass stoppers.
The graduation line is sharp and permanent and there is an easy to read marking spot.

For solutions of solid in a liquid solvent, first dissolve the solid in a vessel other than the volumetric flask, in case the solid must be heated or crushed in order to be dissolved.
After the solid dissolves, pour the liquid into the volumetric flask and use the solvent to rinse all of the solution from the vessel into the flask. However, if the solid is known to be very soluble, it may be added directly to the flask.
Clay Triangles
Clay Pipe Triangle
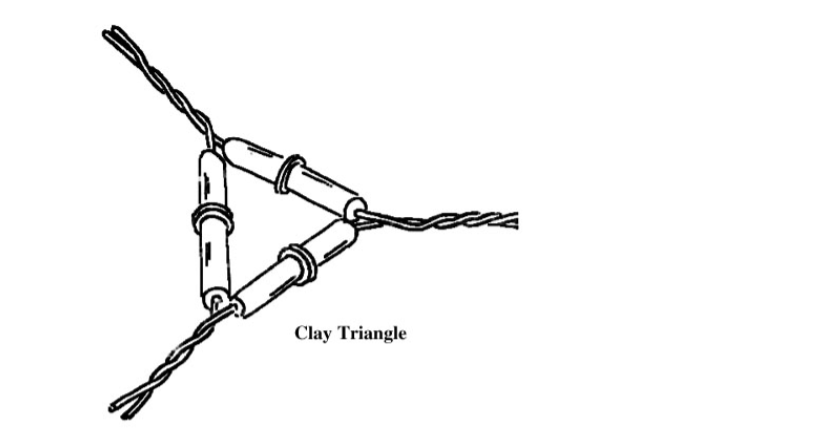
Used for supporting crucibles and dishes when heating on gas or alcohol burners. It has three porcelain pipe stem attached to galvanized iron wire.
Bunsen burner
A Bunsen burner is a laboratory device designed to heat substances for various experiments.
In essence, a Bunsen burner is a small gas burner with an adjustable flame, manipulated at the base by controlling the amount of gas and air admitted into the burner.
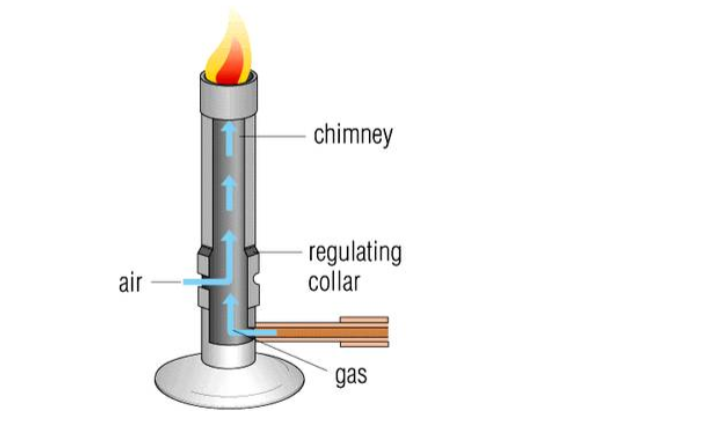
The design of a Bunsen burner includes a vertical metal tube which is connected to a weighted base.
The base includes a nozzle to connect with a fuel source, as well as a gas valve and a flue adjuster to control how much air is admitted through small air holes at the base of the tube.
The gas mixes with air at the bottom of the tube and then rises to the top of the Bunsen burner, where it can be lit with a match or lighter.
In general, the scientist should make sure that hair and clothing are secure, and unlikely to fall into the flame.
In addition, flammable chemicals should be kept away from the Bunsen burner, and someone should remain by the burner at all times to supervise it.
The flexible rubber hose connecting the Bunsen burner to the gas nozzle on the lab bench should also be secure, with no evidence of leaking, and people should be cautious about touching things which have been exposed to the often considerable heat of a Bunsen burner, especially glass objects.
Flame
A typical flame consists of three zones that are easily distinguished.

- The innermost zone, a non-luminous cone, is composed of a gas-air mixture at a comparatively low temperature.
- In the second, or luminous, cone, hydrogen and carbon monoxide are produced by decomposition and begin to react with oxygen to form water and carbon dioxide, respectively.In this cone the temperature of the flame—about 590° to 680° C is great enough to dissociate the gases in the flame and produce free particles of carbon, which are heated to incandescence and then consumed.
The incandescent carbon produces the characteristic yellow light of this portion of the flame.
- Outside the luminous cone is a third, invisible cone in which the remaining carbon monoxide and hydrogen are finally consumed.If a cold object is introduced into the outer portions of a flame, the temperature of that part of the flame will be lowered below the point of combustion, and unburned carbon and carbon monoxide will be given off.
Thus, if a porcelain dish is passed through a candle flame, it will receive a deposit of carbon in the form of soot.
Operation of any kind of flame-producing stove in a room that is unventilated is dangerous because of the production of carbon monoxide, which is poisonous.
All combustible substances require a definite proportion of oxygen for complete burning. (A flame can be sustained in an atmosphere of pure chlorine, although combustion is not complete.)
In the burning of a candle, or of solids such as wood or coal, this oxygen is supplied by the surrounding atmosphere.
In blowpipes and various types of gas burners, air or pure oxygen is mixed with the gas at the base of the burner so that the carbon is consumed almost instantaneously at the mouth of the burner.
For this reason such flames are non-luminous.
They also occupy a smaller volume and are proportionately hotter than a simple candle flame.
The hottest portion of the flame of a Bunsen burner has a temperature of about 1600° C. Such flames have a bluish-green cone in place of the luminous cone.
If the oxygen supply is reduced, such flames have four cones: nonluminous, bluish-green, luminous, and invisible.
Hazard Warning Symbols
Hazardous Material Safety Reference Sheets and Material Safety Data Sheets are produced to remind laboratory users of the potential hazards of the materials at hand. The table below shows some common hazard symbols and some of the chemicals on which these may be found.
ChemicalsWe deal with chemicals all day long. A chemical is any substance used or produced in a chemical process.
Water is a chemical produced in a chemical reaction between hydrogen and oxygen.
It is used for chemical processes of life.
Natural materials, e.g. Cotton, wood are produced by chemical processes of living things during their lives.
Natural materials are also chemicals. Man made or synthetic materials are produced by man.
Plastics and nylon are some synthetic materials. Everything in the universe is made of chemicals. Some have chemical names, as well as more common names.
Can you think of three more chemicals that have similar common names and chemical names?2.0.0 Simple Classification of Substances
Matter
Matter can be classified as either a mixture or a pure substance.
Mixtures contain at least two substances.
If you can see the different parts of the mixture it is called a mixture.E.g. Soil and concrete
Properties of mixtures depend on the proportions of the parts.Have you ever made a cup of coffee and added too much sugar?
Pure substances have properties that are always the same. You can identify an unknown substance by testing its properties, e.g. Gold
ActivityWhich of these substances is/ is not a mixture?
What made it possible for you to determine whether or not it was a mechanical mixture? What about the other substances? Is it hard to tell them apart?Of course.
It is hard to determine whether they are solutions or pure substances by just looking at them.
You must take into consideration all of their physical properties. Remember…Properties are characteristics that you can use to describe or identify different substances!
Can you name three properties that would be useful or helpful in determining whether they are solutions or pure substances?
We can check whether the melting point, boiling point or density on the labels match the information in the table below. If they do match, we can say the substances are more than likely a pure substance.
In your lab you will examine samples of unknown substances. While you examine these substances, you need to try and classify them as either a mixture, a solution or a pure substance.
Make sure you read and then write out the lab rules before you begin.
Remember to follow the safety precautions of working in the lab and have all of your materials prepared before you start!
Lab Activity: Classifying Chemicals With Data Problem:
How can you classify unknown materials as mechanical mixtures, solutions or pure substances?
Hypothesis: How do you think it is going to turn out? Use the facts you already know to come up with a guess that might really make sense.
Materials:
-12 samples of unknown materials
-data table about properties of pure substances (See below).
Table: Properties of Some Pure Substances
Vocabularycompounds, element, periodic table, pure substance pure Substances
There are millions of kinds of pure substances. As scientists began studying substances, they found that there were certain simple substances that were in all of the materials that they studied.
They called these “building blocks of matter” elements.
Elements are pure substances that cannot be broken down to any simpler substance.
Compounds, are substances made up of two or more elements combined in specific proportions.
If each letter in the alphabet is an element, then words would be compounds.
There are only about 100 elements in the universe that we know. Each of these elements has their own specific properties. Some are more common, some rare, some poisonous, some radioactive, and some explosive.
As scientists began studying substances, they found that there were certain simple substances that were in all of the materials that they studied. They called these “building blocks of matter” elements.
Elements are pure substances that cannot be broken down to any simpler substance. Compounds, as we have seen, are substances made up of two or more elements combined in specific proportions.
Ex. Hydrogen + Oxygen = Water (H20)
Carbon + Hydrogen + Oxygen = Alcohol Elements, Compounds and the Atomic Theory
Vocabulary
atomic theory, atom, bonds, chemical formula, chemical symbol, compound, element, matter, mixture, molecule, particle theory, proportions
Elements, Compounds and the Atomic Theory
Atoms are the base of chemistry! They are the base for everything in the Universe! Matter is composed of atoms.
The Particle Theory states that all matter is made of particles.
The Atomic Theory goes farther to say that there is a difference between elements and compounds. Atoms are the smallest particles of elements.
Since there are about 100 elements, there are about 100 kinds of atoms. Atoms can join together in many combinations to form molecules.
If the atoms of a molecule are the same, the substance is an element.
If the atoms of a molecule are different, the substance is a compound.
Classify each item as either an element (E), a compound (C), or a mixture (M). Use this site’s glossary for definitions of these terms.
You may also use the periodic table to help you identify elements.
• water
• hydrogen
• soup
• soil
• diamond
• sugar
• sulphur
• iron sulfide
• mercury
• nitrogen
• salt
• bread
• gold
• aspirin
• iron
• brass
• sausage
• cement
• oxygen
• human body
• chop suey
• air
Compounds and Their Proportions
All elements are represented by a chemical symbol. It is either a single capital letter, or a capital letter followed by a small letter.
Examples: Ca = calcium Cu = copper
C = carbon N = nitrogen
Combinations of symbols represent compounds. These compounds are called chemical formulas.
Example: H20 2 hydrogen atoms
1 oxygen atom
If no number is shown beside the symbol, a 1 is understood.
If more than 1 atom is present, a small number is shown after the atom to indicate how many atoms are in the compound.
NaHCO3 = Sodium Hydrogen Carbonate (baking soda) 1 atom Na = Sodium
1 atom H = Hydrogen
1 atom C = Carbon
3 atoms O = Oxygen
1. Matter
Matter is:
• Anything that has mass and occupies space (has volume)
• Composed of particles (molecules, ions, atoms).
These are in constant motion attracting one another with inter-particle forces (or cohesive)
• Is a solid, liquid or gas depending on interparticle forces of attraction and spaces between particles.
Matter has many properties. It can have physicaL properties like different densities, melting points, boiling points, freezing points, color or smells.
There are also chemical properties that define matter.
A good example of chemical properties is the way elements combine with each other in reactions. Matter can change in two major ways, physically and chemically.
Physical changes
These are changes that do not result in the production of a new substance.
Only the physical state of the material changes.
The substance retains exactly the same chemical composition. If you melt a block of ice, you still have water at the end of the change.
If you break a bottle, you still have glass.
Some common examples of physical changes are; melting, freezing, condensing, breaking, crushing, cutting, and bending.
Special types of physical changes where any object changes state, such as when water freezes or evaporates, are sometimes called changes of state.
Chemical Changes
These are changes that result in the production of anew substance. When you burn a charcoal in a fireplace, you are carrying out a chemical reaction that releases carbon dioxide.
When you light a candle, you are carrying out a chemical reaction that produces water and carbon dioxide.
Common examples of chemical changes that you may be familiar with are; digestion, respiration, photosynthesis, burning, and decomposition.
Signs that a chemical reaction has happened include:
a) Colour changes,
b) Temperature changes
c) Change in mass
Examples of changes
Physical changes
1. Even at room temperature bottles of solid iodine show crystals forming at the top of the bottle above the solid.
The warmer the laboratory, the more crystals form when it cools down at night!
I2 (s) I2 (g) (physical change only)
2. Solid carbon dioxide (dry ice) is formed on cooling the gas down to less than -78oC. On warming it changes directly to a very cold gas, condensing any water vapour in the air to a ‘mist’, hence its use in stage effects.
Physical and chemical changes
CO2 (s) CO2 (g) (physical change only)
On heating strongly in a test tube, the white solid ammonium chloride decomposes into a mixture of two colourless gases ammonia and hydrogen chloride.
On cooling the reaction is reversed and solid ammonium chloride reforms at the cooler top of the test tube.
Ammonium chloride + heat ammonia + hydrogen chloride
NH4Cl(s) H3(g) + HCl(g)
This reaction involves both chemical and physical changes.
Kinetic Particle Theory
• Matter is made up of particles that are in constant motion
• The higher the temperature, the faster the particles move (more energy)
• Increase in temperature increase weakens interparticle forces, causing particles to spread apart and increase in volume/size (i.e. Expansion)
• Gases have greatest average energy while solids have smallest average energy
There are three physical states of matter.
• Solid
• Liquid
• Gas
Solids
• Have closely packed particles• Have definite shape and volume
• Have particles that vibrate about fixed positions
• When heated, particles vibrate more vigorously, bonds weaken, particles space out and solid expands.
Liquids
Liquids:• Flow freely because their particles slide over each other as they have weak inter-particle forces.
• Have no definite shape
• Have definite volume cannot be squashed
• Can flow because inter-particle forces between liquid particles are weak and so the particles can slide over/past each other.
Gases
Gases;• Offer least resistance
• Occupy greater volume than same mass of solids/liquids
• Have particles that are widely spaced apart (weak inter-particle forces) and move with great speed
• No fixed volume, no fixed shape
• Are only restricted by shape and size of container
• Particles are far apart and can be pushed together (can be easily compressed)
• Move around easily, quickly and randomly colliding with each other and bounce off, spacing out.
Summary of properties of matter
<bChanges of State and the Kinetic TheoryWe can use the state particle models, and the diagrams shown below, explain changes of state and the energy changes involved.
Evaporation and Boiling (liquid to gas)
</b
- On heating particles gain kinetic energy and move faster.
- In evaporation and boiling the highest kinetic energy molecules can ‘escape’ from the attractive forces of the other liquid particles.
- The particles lose any order and become completely free to form a gas or vapour.
- Energy is needed to overcome the attractive forces in the liquid and is taken in from the surroundings.
- This means heat is taken in, so evaporation or boiling are endothermic (require heat to be added) processes.
- If the temperature is high enough boiling takes place.
- Boiling is rapid evaporation anywhere in the bulk liquid and at a fixed temperature called the boiling point and requires continuous addition of heat.
- The rate of boiling is limited by the rate of heat transfer into the liquid.
- Evaporation takes place more slowly at any temperature between the melting point and boiling point, and only from the surface, and results in the liquid becoming cooler due to loss of higher kinetic energy particles.Condensing (gas to liquid)
- On cooling, gas particles lose kinetic energy and eventually become attracted together to form a liquid.
- There is an increase in order as the particles are much closer together and can form clumps of molecules.
- The process requires heat to be lost to the surroundings i.e. heat given out, so condensation is exothermic.This is why steam has such a scalding effect, it’s not just hot, but you get extra heat transfer to your skin due to the exothermic condensation on your surface!
Melting (solid to liquid)
When a solid is heated the particles vibrate more strongly as they gain kinetic energy and the particle attractive forces are weakened.
- Eventually, at the melting point, the attractive forces are too weak to hold the particles in the structure together in an ordered way and so the solid melts.
- The particles become free to move around and lose their ordered arrangement. Energy is needed to overcome the attractive forces and give the particles increased kinetic energy of vibration.
- So heat is taken in from the surroundings and melting is an endothermic process.
- Freezing (liquid to solid)On cooling, liquid particles lose kinetic energy and so can become more strongly attracted to each other.
Eventually at the freezing point the forces of attraction are sufficient to remove any remaining freedom and the particles come together to form the ordered solid arrangement.
Since heat must be removed to the surroundings freezing is an exothermic process.
Cooling and Heating Curves
Sublimation:This is when a solid, on heating, directly changes into a gas, and the gas on cooling re-forms a solid directly.
Theory in terms of particles:
When the solid is heated the particles vibrate with increasing force from the added thermal energy.
If the particles have enough kinetic energy of vibration to partially overcome the particle-particle attractive forces you would expect the solid to melt.
However, if the particles have enough energy at this point that would have led to boiling, the liquid will not form and the solid turns directly into a gas.
Overall, this is an endothermic change as energy absorbed and ‘taken in’ to the system. On cooling, the particles move slower and have less kinetic energy.
Eventually, when the particle kinetic energy is low enough, it will allow the particle-particle attractive forces to produce a liquid.
But the energy may be low enough to permit direct formation of the solid, i.e. the particles do not have enough kinetic energy to maintain a liquid state! Overall this is an exothermic change, energy released and ‘given out’ to the surroundings.
Summary
• Anything that has mass and occupies space (has volume)
• Matter is composed of particles (molecules, ions, atoms)
Spaced apart and seen with scanning electron microscope
• Are in constant motion attracting one another with inter-particle forces (or cohesive)
• Strength of interparticle force and space between particles determines the state.
Conductors and Insulators
The ability to conduct electricity is the major simple distinction between elements that are metals and non-metals.
Electrical Conductors
A conductor is a material that conducts electricity but is not chemically changed in the process.
They carry an electric current through freely moving electrons when a potential difference is applied across them. They include:
- All metals (molten or solid) and the non-metal carbon (graphite). This conduction involves the movement of free or delocalized electrons (e- charged particles) and does not involve any chemical change.
- Any molten or dissolved material in which the liquid contains free moving ions is called the electrolyte. Ions are charged particles eg Na+ sodium ion, or Cl- chloride ion, and their movement or flow constitutes an electric current, because a current is moving charged particles.All metals and graphite are conductors of electricity.
InsulatorsAn insulator is a material that does not conduct electricity. Such materials have no free electrons.
Electrolysis
Electrolysis is the process of electrically inducing chemical changes in a conducting melt or solution eg splitting an ionic compound into the metal and non-metal.
Summary of Common Electrical Conductors
These materials carry an electric current via freely moving electrically charged particles, when a potential difference (voltage) is applied across them, and they include:
1) All metals (molten or solid) and the non-metal carbon (graphite).
This conduction involves the movement of free or delocalised electrons (e- charged particles) and does not involve any chemical change.
2) Any molten or dissolved material in which the liquid contains free moving ions is called the electrolyte. Ions are charged particles eg Na+ sodium ion, or Cl- chloride ion, and their movement or flow constitutes an electric current, because a current is moving charged particles.
The movement of opposite charges during electrolysis is due to the attracting in the electric field produced by the potential difference (the voltage).
Liquids that conduct must contain freely moving ions to carry the current and complete the circuit.
You can’t do electrolysis with an ionic solid! The ions are too tightly held by chemical bonds and can’t flow from their ordered situation! When ionically bonded substances are melted or dissolved in water the ions are free to move about.
However some covalent substances dissolve in water and form ions. eg hydrogen chloride HCl, dissolves in water to form ‘ionic’ hydrochloric acid H+Cl-(aq).
Conductors Non-conductors
All metals (copper, iron, lead, magnesium etc) and graphite, a non-metal.
Most non-metals (sulphur, iodine,charcoal etc.) Most solid compounds (All gases are non-conductors)
Liquids are classified in three groups as regards their conductivity of electricity:
(i) Those that pass an electric current and are not decomposed by if (conductors)
(ii) Those that pass an electric current and are decomposed by it (electrolytes)
(iii) Thos that do not pass an electric current (non-electrolytes).
Conductivity of liquids
In summary, the following substances are electrolytes:
- Molten salts
- Solutions of salts in water
- Solutions of acids
- Solutions of alkalisMetallic conductivity:
- Electrons flow(carry charge)
- It is a property of elements, graphite and alloys
- It takes place in solids and liquids
- No chemical change takes place.Electrolytic conductivity:
- Ions flow (carry charge)
- It is a property of ionic compounds
- Takes place in liquids (molten salts) and solutions but not solids
- Chemical decomposition takes place.Simple Classification of Substances
Pure Substance
Pure means that only one substance is present in the material and can be a pure element or compound.
A simple physical test for purity, and properties that can help identify a substance, is to measure the boiling point or melting point.
Every pure substance melts and boils at a fixed temperature.
If a liquid is pure, it should boil at a constant temperature called the boiling point e.g. water boils at 100oC.
An impure liquid will boil at a higher temperature if it contains a dissolved solid impurity e.g. seawater, containing dissolved salts, boils at over 100oC.
The boiling then takes place over a range of temperatures. If a solid is pure, it melts sharply at its fixed melting point.
An impure solid melts below its expected melting point and the more impure, the wider the temperature melting range, e.g. a water and salt mixture melts below 0oC.
Impure usually means a mixture of mainly one substance plus one or more other substances physically mixed in.
The purity of a compound is important, particularly in drug manufacture.
Any impurities present may be harmful substances.
Mixtures
A mixture is a substance made up of at least two substances which may be elements or compounds.
They are usually easily separated by physical means e.g. filtration, distillation, chromatography etc.
Separation methods are needed to purify materials and separate useful materials. Pure substances are rare.
Most of the things we handle or interact with are impure. Think of the air we breathe in it is not pure.
It is a mixture of gases. The other gases in air are useful one way or the other.
Distinction between Compounds and Mixtures
The following example of iron, sulphur and iron sulphide will make us understand the difference between mixtures and compounds.Properties of Iron and Sulphur
The following experiment shows the difference in properties between the elements iron and sulphur.
Preparation of a Mixture of Iron and SulphurTake about 7g of freshly powdered iron, and a little more than 4 g of sulphur. Mix them well. However well it may be mixed, the iron and sulphur particles can be seen separately.
Also the above said properties of both iron and sulphur will still be exhibited by this mixture.
Preparation of Iron Sulphide
Take this mixture of iron and sulphur in a hard glass test tube. Heat over spirit lamp or burner.
The sulphur starts melting. Then the mixture catches fire. Stop heating. The flame spreads throughout the mixture, evolving heat.
When it dies out, heat the test tube very strongly, so that any extra unreacted sulphur gets burnt out.
Dip the red-hot test tube in cold water. The test tube breaks. Collect the iron sulphide formed, and powder it.
Compare the properties of the mixture of iron and sulphur with that of the iron sulphide formed.
Comparison of properties of a mixture of iron and sulphur with iron sulphide is shown below.Comparison of properties of a mixture of iron and sulphur with iron sulphide
Solutes and solventsWhen a salt is mixed with water and the mixture stirred, a solution is produced.
The salt is the solute, water is the solvent and the resulting mixture is called a solution.
Solute – A substance that will dissolve in a liquid e.g. salt.
Solvent – A liquid into which a substance dissolves e.g. water.
Solution – Is a uniform mixture of solute and solvent.
Miscible and Immiscible substances
1. Miscible: These are substances capable of being mixed. They are soluble in each other. Water and alcohol are miscible as they dissolve to make a solution.
2. Immiscible: These are substances that cannot be mixed. They do not dissolve in each other. Oil and water are immiscible. They separate into two layers, e.g. paraffin and water.
Homogeneous and Heterogeneous mixtures
Water and alcohol are completely miscible.
They make a homogenous solution. In other types of mixture, the state remains separate.
This is known as an heterogeneous mixture. There are a good number of substances that make hetegeneous mixtures.
(a) Heterogeneous mixtures
(b) Homogeneous Mixtures (solutions)
Separation of MixturesSometimes we need pure substances as opposed to impure ones. You can imagine a mixture of sand and table salt and imagine how much use you would have for it.
Or imagine the manufacture of drugs and medicines.
Purity is highly essential here as it is crucial to our well-being and health.
A range of physical techniques are available to make necessary separations.
All separations depend one way or other on the physical properties of substances in the mixture.
The method chosen depends on.
(i) The type of mixture
(ii) Substance in the mixture which we are interested in.
Types of Mixtures and Separation
Purity based on melting/Boiling Points• Substances can be identified using their boiling and melting points
• Pure substances change state at a constant temperature whilst impure substances change over a range of temperature.
• If a solid is not pure, its melting point will be low and its boiling point will be high.
• Impurities widen the range over which the substance is liquid.
• The surrounding pressure can increase and decrease boiling and melting points.
If the surrounding pressure falls, the boiling point falls. An increase in pressure raises the boiling point.
• No two substances have the same boiling point and same melting point
Separation Techniques
1. Separating Funnel
• Used to separate liquids that are immiscible (form layers on one another).
• When a mixture of oil and water is poured into the funnel, the oil floats on top. When tap opened, water runs out and closed when layer of water finished.
2. Filtration• Used to mainly separate suspensions, e.g. chalk and water or sand and water.
• Filter paper is aligned around the inner surface of a filter funnel and the solution is passed through.
• The solute (suspension) particles are trapped by the filter paper as residue
• The solvent passes through the filter paper and collects as filtrate
3. Evaporation• Used to separate solutions usually with a solute that consists of particles, which have been spread throughout the solvent(dissolved) and are too small to be obtained in filtration.
• Solution heated and solvent evaporates leaving solid behind.
• Salt obtained from solution by this method
4. Crystallization• Solids dissolved in solution can be separated out by letting them form crystals (E.g. copper (II) sulphate). The crystals contain some water of crystalisation.
• When a solution of copper (II) sulphate is cooled, then crystals of the salt form because it is less soluble at a lower temperature.
• In crystallization, a solution of copper (II) sulphate is heated so that some of the water evaporates leaving a more concentrated solution of the salt.
• The solution can be checked to see if it is ready by placing one drop on a microscopic slide, and crystals should form on the cool glass.
• The solution is then left to cool and crystallize. The crystals are removed by filtering, rinsed with water and dried with filter paper.
5. Fractional Distillation
• Used to separate two miscible liquids that have different boiling points e.g. a mixture of ethanol and water.
• Mixture is heated; at 78°C ethanol begins to boil. Some water evaporates too.This mixture of ethanol vapour and water vapour condenses in the cool glass beads in the column, making them hot.
• When the glass beads reach 78°C, ethanol vapour no longer condenses on them, only water vapour does. The water vapour then drips back into the flask, whilst the ethanol rises through into the condenser.
• The cool condenser forces ethanol to condense (liquid ethanol) and drip into the beaker.
• When the thermometer rises above 78°C, it is a sign that ethanol has been separated so heating can stop.
6. Distillation
• Method used to obtain pure solvent from a solution, e.g. obtaining pure water from salt water
• Solution heated in a round bottomed flask A. It boils and steam rises into condenser, leaving salt behind.
• A thermometer is placed above the mixture so as to control the temperature at which distillation occurs.
• Condenser is cold and steam condenses into water, which drips into beaker completely pure (distilled water).
7. Paper Chromatography• Used when chemists want to analyse a mixture (find out what substances are present in it), e.g. finding out what dyes are present in ink.
• A pencil line is normally drawn at the point where the ink drop is put to mark the starting point. Ink must never be used as it blots and messes up the final result.
• When a drop of solution applied to paper, the paper absorbs solute/binds it to surface. As the solvent rises, some solute stays put and others dissolve in the solvent and travels up the paper.
• The most soluble dye travels through the paper faster than one which is slightly soluble.
• When the solvent reaches the top of the paper, the process stops and different spots are left on the paper. The point where the solvent stops is known as the solvent front.
• Each spot represents another solute, this way they are separated.
• Many solvents used, ethanol or propanone.
• With a solvent other than water, a closed container should be used so that the vapour of the solvent surrounds the Chromatography paper.
• Can be used to separate a mixture of coloured substances (e.g. in black ink)
• On a circular filter paper, substances more soluble will form larger circles with least soluble forming smaller circles.
• Chromatography can also be used to separate substances that are not coloured and cannot be seen with our naked eyes. For this to be possible, the paper is treated with a locating agent after the chromatography run. The agent reacts with samples to produce coloured spots.Constituents of Matter
Atoms, Molecules, Elements and Compounds
The Atom
An atom is the smallest particle of a substance which can have its own characteristic properties.
Atoms are built up of even more fundamental sub-atomic particles. These are electrons, protons and neutrons.
The protons and neutrons are in the nucleus (centre) of the atom and the electrons orbit round the outside in shells (energy levels or layers).So you will often see pictures of atoms that look a little like this:
How many protons, neutrons and electrons does an atom have?You can work this out using the periodic table. Every element in the periodic table has two numbers with it: the atomic number and the mass number.
For example for lithium, the numbers are:
The atomic number is the number of protons that the atom has. It is also the number of electrons that the atom has. So lithium has 3 protons and 3 electrons.The mass number is the number of protons and neutrons added together. So, for lithium there are 7 protons and neutrons combined, and we know that 3 of them are protons so there must be 4 neutrons.
The atomic number (Z) is also known as the proton number of the nucleus of a particular element. It is the proton number that determines the specific identity of a particular element and its electron structure. The mass number (A) is also known as the nucleon number,that is the sum of neutrons and protons in the nucleus of an atom.
The neutron number (N) = mass number (A) – proton/atomic number (Z)
Protons and neutrons are the nucleons present in the positive nucleus and the negative electrons are held by the positive nucleus in ‘orbits’ called energy levels or shells. In a neutral atom the number of protons equals the number of electrons.
Example.
How many electrons, protons and neutrons are present in an atom of sodium?
(a) Sodium has mass number 23 and atomic number 11
Number of electrons = atomic number = 11
Number of protons = atomic number = 11
Number of neutrons = mass number – atomic number
= 23 – 11
= 12
Table of mass number, atomic number and symbol of selected elements
SummaryAtoms:
- Are made up of protons, neutrons and electrons
- Are the smallest units or building blocks of elements
- Take part in chemical reactions
- Of the same element are the same
- Of different elements are different due to different numbers of protons, neutrons and electrons
- Have equal number of electrons and protonsElements and Symbols
An element is a pure substance made up of only one type of atoms. About 92 in the Periodic Table naturally occur from hydrogen H to uranium U.
Note that each element has symbol which is a single capital letter like H or U or a capital letter + small letter e.g. cobalt Co, calcium Ca or sodium Na.
Each element has its own unique set of properties but the Periodic Table is a means of grouping similar elements together.
They may exist as atoms like the Noble Gases e.g. helium (He) or as molecules e.g. hydrogen (H2) or sulphur S8.
All the atoms of the same element have the same atomic or proton number.
This number determines how many electrons the atom has, and so ultimately its chemistry.
Common ElementsYou should know the name and symbol for the following elements. If you see the name, you should know the symbol.
If you see the symbol, you should know the name. For the elements, there are other names for the element, sometimes Latin, from which the element symbol was derived or some other name that makes the element more recognizable.
You do not need to know the names in parentheses.
a. Table of elements whose symbol is the first letter
b. Table of elements whose symbol is first letter and another letter in the name
Compounds and FormulaA compound is a pure substance formed by chemically combining at least two different elements. Compounds are two or more different elements combined. Their atoms have been joined or bonded together.
Compounds can be represented by a FORMULA.
There must be at least two different types of atom (elements) in a compound. Compounds have a fixed composition and therefore a fixed ratio of atoms represented by a fixed formula, however the compound is made or formed.
In a compound, the elements are not easily separated by physical means, and quite often not easily by chemical means either.
A compound has properties quite different from the elements it is formed from. For example, soft silvery reactive sodium + reactive green gas chlorine colourless, not very reactive crystals of sodium chloride.
Chemical word equationsFor any reaction, what you start with are called the reactants, and what you form are called the products. So any chemical equation shows in some way the overall chemical change of.
REACTANTS -> PRODUCTS
This can be written in words or symbols/formulae. The arrow means the direction of change from reactants =to=> products No symbols or numbers are used in word equations.
Always try to fit all the words neatly lined up from left to right, especially if it is a long word equation.
The word equation is presented to summarise the change of reactants to products.
Here are some word equations
Iron + sulphur -> iron sulphide
Sodium hydroxide + hydrochloric acid -> sodium chloride + water
Magnesium + hydrochloric acid -> magnesium chloride + hydrogen
Magnesium hydroxide + nitric acid -> magnesium nitrate + water
Acids, Bases and Indicators
Introduction
All the elements in nature fall into three classes: metals, non-metals and gases. Non-metals are also sometimes called metalloids.
The compounds formed by combination of the elements can also be classified as organic and inorganic compounds.
Organic compounds are formed from a combination of carbon and hydrogen; they are also sometimes known as hydrocarbons.
In addition to this, all these compounds taste sour, bitter or salty.
The sour tasting substances are known as acids. Bitter tasting compounds are generally soapy to feel also; they are known as bases or alkalis.
What is an acid?
When a substance dissolves in water, the solution may be acidic, neutral or alkaline. An acid is any substance which produces H+ ions or H3O+ ions in water. H+ ions are called hydrogen ions; H3O+ ions are called hydroxonium ions. You will mostly see acids in reactions as forming H+ ions. In reality, H+ is a single proton, and does not exist on its own.
It always attaches to something; in water it joins to H2O to form H3O+ ions. All acids taste sour and are mostly derived from oxides of non-metals dissolved in water.
Measure of acidity pH.PH is a measure of how acidic or how alkaline a solution in water is.
The pH scale goes from 1 to 14, with 1 being very strongly acidic, and 14 being very strongly alkaline.
A pH of 7 is neutral. You can measure the pH of a solution using universal indicator.
Just as litmus paper will be red for an acid and blue for an alkali, so universal indicator is a mixture of indicators which will give different colours for a different pH.
Any acid will have a pH of less than 7. Any alkali will have a pH of more than 7.
A strong acid (HCl or H2SO4 or HNO3 )will have a pH of 1 (red).
A weak acid will have a pH of 3 to 4 (orange). Examples of weak acids are ethanoic acid (vinegar), citric acid (lemon juice) and rain water.Rain water has a natural pH of 5•5 (see carbonic acid). Water and salts are neutral, pH 7 (green).
A weak alkali (ammonia) will have a pH of 11 to 12 (blue). A strong alkali (Ca(OH)2 or NaOH) will have a pH of 14 (purple).
Examples of Acids.
The three common acids you will find in the laboratory are
1) Hydrochloric acid – HCl(aq)
2) Nitric acid – HNO3(aq)
3) Sulphuric acid – H2SO4(aq)
They are all strong acids – see pH. They all ionise in water to form hydrogen ions (H+ ions).
1) HCl(aq) H+(aq) + Cl-(aq)
2) HNO3(aq) H+(aq) + NO3–(aq)
3) H2SO4(aq) H+(aq) + HSO4–(aq)
They are all examples of hydrogen compounds with non-metals. Hydrochloric acid is hydrogen chloride (in water).
Nitric acid is hydrogen nitrate (in water). Sulphuric acid is hydrogen sulphate (in water).
Sulphuric acid is made using the contact process. They are called Mineral Acids because they were originally obtained from minerals in rocks.Oxides of non-metals are acidic, such as CO2, NO, SO2.
Hydrogen oxide (H2O) is Water – it is neutral, see Water. Lots of everyday substances contains acids.
Acids are found in:
- citrus fruits (le mon juice, orange juice)
- vinegar
- car batteries (sulphuric acid)
- your stomach (hydrochloric acid)Rainwater is a little acidic, but pollution (e.g. sulphur dioxide) from burning fossil fuels may make it even more acidic, forming acid rain.
When acids are present in food, they usually taste sour (think of the taste of lemon juice or vinegar).
Strong acids are very dangerous.
Properties of Acids.
They have a pH less than 7, see pH. They will turn blue litmus paper red. Hydrochloric acid and sulphuric acid will react with.
- Any alkali or base, see neutralisation.
- Any metal above hydrogen in the reactivity series. The metal will fizz, giving off hydrogen gas, and leaving the metal salt in solution.It is not safe to put a metal into an acid which is above magnesium in the reactivity series.
- Any chloride or sulphate can be safely made by reacting the appropriate metal (from lead to magnesium in the reactivity series) with hydrochloric acid to make the chloride or sulphuric acid to make the sulphate.
- Any metal carbonate or metal hydrogen carbonate. The metal carbonate or metal hydrogen carbonate will bubble giving off carbon dioxide gas, leaving the metal salt and water.Any chloride or sulphate can be made by reacting the appropriate metal carbonate or hydrogen carbonate with hydrochloric acid to make the chloride or sulphuric acid to make the sulphate.
Strong and Weak Acids – Strength and Concentration.
Acids and alkalis can be described as strong or weak. This does not mean the same as concentrated or dilute.
The strength of an acid or alkali depends on how ionised it is in water.
A strong acid or alkali is completely (100%) ionised. For hydrochloric acid
hydrogen chloride (in water) hydrogen ion + chloride ion HCl<sub(aq) <=”” sub=””> H+(aq) + Cl–(aq) All of the hydrogen chloride molecules become hydrogen ions and chloride ions in water (see examples for other strong acids).</sub(aq)>
For sodium hydroxide
sodium hydroxide (in water) sodium ion + hydroxide ion
NaOH(aq) Na+(aq) + OH–(aq)
Sodium hydroxide exists as ions both in water and in the solid.
(see examples for other strong alkalis).
A weak acid or alkali is only partly (less than 100%) ionised. For ethanoic acid
ethanoic acid (in water) hydrogen ion + ethanoic ion
CH3CO2H(aq) H+(aq) + CH3CO2–(aq)
Some of the ethanoic acid molecules become ions in water but most of them stay as molecules. The reaction is reversible (shown by the arrow).
Ammonia
ammonia + water ammonium ion + hydroxide ion NH3(g) + H2O(l) NH4+(aq) + OH–(aq)
L Some of the ammonia molecules become ions in water but most of them stay as molecules. See also Concentration and Differences between Strong and Weak Acids.
Common uses of Acids – see also uses of Sulphuric Acid.
Outside of their uses in the chemical industry,
common uses of acids are
- Steel used in construction is acid treated before painting. Dilute sulphuric or hydrochloric acid will remove any surface rust which would otherwise spread under the painted surface. ‘Rust remover’ used to repair cars is dilute phosphoric acid – H3PO4.
- Baking powder contains tartaric acid.
- ‘Lime scale’ removers contain dilute acids. Try using lemon juice or vinegar (weak acids). Lime scale is calcium carbonate (also called furring).
- A wasp sting is alkaline. It may be neutralised with a weak acid (lemon juice or vinegar).
- A bee sting is acidic. It may be neutralized by an alkaliWhat is an alkali?
Alkali is pronounced like alcohol, with ‘lie’ at the end instead of ‘hol’. An alkali is any substance which produces OH– ions in water. OH– ions are called hydroxide ions.
If there are excess of (OH)– ions when a compound is dissolved in water, the solution is called a base or an alkaline solution.
A base is generally a metal hydroxide solution.
Table below lists some of the common alkalis available in our everyday lives. Name of alkali Chemical Formula Dissociation in water Sodium Hydroxide NaOH Na+ + (OH)– Potassium Hydroxide KOH H+ + NO3– Ammonium Hydroxide NH4OH NH4+ + (OH)– A substance which will neutralize an acid, but does not dissolve in water, is called a base. For example, copper (II) oxide, iron (II) oxide and zinc carbonate are bases. They do not dissolve in water.
Any base which dissolves in water is called an alkali.
The outer circle encloses all bases, while the inner circle selects those which are alkalis or soluble bases.Examples of Alkalis.
The three common alkalis you will find in the laboratory are
1) Sodium Hydroxide solution – NaOH(aq).
2) Calcium Hydroxide solution – Ca(OH)3(aq), (lime water)
(Lime water is used in the test for carbon dioxide).
3) Ammonia solution – NH3(aq).
1 and 2 are strong alkalis, 3 is a weak alkali – see pH.
They all ionise in water to form hydroxide ions (OH- ions).
- 1) NaOH(aq) Na+(aq) + OH-(aq)
- 2) Ca(OH)2(aq) Ca2+(aq) + 2OH-(aq)
- 3) NH3(aq) + H2O(l) NH4+(aq) + OH–(aq) NH4+ is an ammonium ion.Any metal oxide or hydroxide is a base.
If the base dissolves in water it is called an alkali.
Alkalis are found in:
- oven cleaner (sodium hydroxide)
- soap
- cleaning fluid e.g. spray-and-wipe (ammonia) Notice the connection between these substances? Alkalis are often found in substances for cleaning.Strong alkali substances are just as dangerous as strong acidic substances, causing very serious burns if they come into contact with your skin.
Properties of Alkalis.
They have a pH greater than 7, see pH.
They will turn red litmus paper blue.
They will react with acids to form a salt and water, see neutralisation.
Uses of Alkalis.
- Sodium hydroxide is used in the manufacture of paper, soap and ceramics.
- Calcium hydroxide (called ‘slaked lime’, or just ‘lime’), is added to soils or lakes to make them less acidic (see acid rain).
- Indigestion may be caused by too much hydrochloric acid in the stomach. Indigestion tablets contain a base such as magnesium oxide, or calcium carbonate to neutralise the acid.
- A bee sting is acidic. It may be neutralised by a weak alkali such as ammonia solution.The pH values of some common solutions are shown in the table below.
Weak, strong acids and alkalis
Water.In a sample of water,a very small number of water molecules will form ions. Water hydrogen ion + hydroxide ion. H2O(l) H+(aq) + OH–(aq)
This ionisation is reversible (shown by the arrow).
The hydrogen ion is acidic. The hydroxide ion is alkaline. Water forms equal amounts of both ions, and so water is neutral. Compare this reaction with neutralisation.
Indicators
Red cabbage juice solution works well instead of universal indicator solution.
Making Cabbage Indicator
Acid base indicators are chemicals that change colour in the presence of different pH levels. These are usually larger organic molecules.
Some, like that in purple cabbage, are natural.
You will be making an acid base indicator from purple cabbage. This indicator is a very good one with good color changes.
Materials Needed:
Tea strainer
2 Glass quart jars with lids
1 Quart distilled water
uncooked purple cabbage
Hotplate and pan
Procedure:
a. Fill one jar with cabbage leaves that have been crushed into small pieces.
b. Heat the distilled water to boiling, and fill the jar containing the pieces of cabbage with the hot water.
c. Allow the jar to stand until the water cools to room temperature.
d. Poor the cooled cabbage solution through the tea strainer into the second quart jars. Discard the cabbage leaves.
e. Store the cabbage indicator in a cool place until needed.
Results:
The hot water dissolved the colored chemicals in the cabbage.
These colored chemicals turn red when mixed with an acid, and green when mixed with a base.
This indicator can be used to test for the presence of either an acid or a base.
Indicator Colors
Air and CombustionPercentge of Oxygen in Air
We have read that Air consists mainly of molecules of oxygen and nitrogen with important yet trace amounts of other gases.
We know that the combustion of organic material requires oxygen.
The idea here is to capture a quantity of air in a measured and isolated volume and then use up all the oxygen by burning something.
The remaining volume will be mostly molecular nitrogen.
Equipment needed
1. flasks
2. water container
3. candles
4. rulers
5. thermometer
Procedure
a. The volume of a flask is measured.
b. Water is placed in a container along with a thermometer, and a flask inverted over a lit candle resting in the water. Eventually the candle goes out.
c. Measure the height of water relative to the original water height. Calculate the volume.
d. From the total volume, assumed to be oxygen and nitrogen, compute the percentage of oxygen.
Suppose the heights of air were measured before and after several days and the following measurements were madeHeight of air at the start of experiment = 100cm
Height of air after several days = 80cm
Percentage oxygen in the air = Change in volume of air X 100%
Total volume of air
= 100cm – 80cm X 100%
100cm
= 20%
The approximate composition of the air today is
The proportion of carbon dioxide in the air has risen from 0•03% to 0•04% in the last hundred years due to the burning of fossil fuels.Rusting of Iron
Corrosion
The eating up of metals by the action of air and moisture on their surface is called corrosion.
The corrosion of iron is called rusting. While other metals are said to ‘corrode’, iron metal is said to ‘rust’.
Rusting of Iron
When an iron object is left in damp air (or water) for a considerable length of time, it gets covered with a red-brown flaky substance called rust. This is called rusting of iron.
Conditions Necessary for the Rusting of Iron
Rusting of iron (or corrosion of iron) needs both, air and water. Thus, two conditions are necessary for the rusting of iron to take place:
1. Presence of air (or oxygen)
2. Presence of water (or moisture)
The chemical composition of rust is hydrated iron (iii) oxide, Fe2O3. xH2O
Experiment to show that Rusting of Iron Requires Both, Air and Water
We take five test-tubes and put on clean iron nail in each.
a. In the first test-tube, the iron nail is put in tap water without a cork so that it is in contact with air and water.b. In the second test-tube containing iron nail, we put some anhydrous calcium chloride and close its mouth with a tight cork. The anhydrous calcium chloride is added to absorb water (or moisture) from the damp air present in the test-tube and make it dry.
In this way, the iron nail in the first test-tubes is kept in dry air (having no water vapour in it).
c. In the third test-tube containing iron nail, we put boiled water. Boiled water does not contain any dissolved air (or oxygen) in it (This is because the process of boiling removes all the dissolved air from it).
A layer of oil is put over boiled water in the test tube to prevent the outside air from mixing with boiled water. In this way, the iron nail is kept in air-free, boiled water.
The mouth of this test-tube is closed with a cork and it is kept aside for about one week.
d. In the fourth test-tube containing an iron nail, oil is added so that the nail is in contact with neither air nor water.
e. In the fifth test-tube, the nail is placed in sea water so that it is in contact with air, salt and water.
After one week, we observe the iron nails kept in all the three test-tubes, one by one. We find that:
a. No rust is seen on the surface of iron nail kept in dry air (water-free air) in the second test tube. This tells us that rusting of iron does not take place in air alone.
b. No rust is seen on the surface of iron nail kept in air-free, boiled water in the third test tube. This tells us that rusting of iron does not take place in water alone.
c. No rust is seen on the surface of iron nail kept in oil in the fourth test tube, where the nail is in contact with neither air nor water
d. Red-brown rust is seen on the surface of iron nail kept in the presence of both air and water together in the first test-tube.
e. The nail in the fifth test-tube in contact with both air and salty water is very rusty.
f. This tells us that rusting of iron takes place in the presence of both air and water together.
How to stop things rusting
Stopping air and water from reaching iron and steel can prevent rusting. Putting a thin layer on the surface of the iron or steel can stop air and water from reaching iron and steel.
This table show methods used to prevent rusting:
Why do cars in Mombasa rust faster that in Nairobi?High Humidity – Explanation:
- The primary reason why cars in Mombasa rust faster than those in Nairobi is because the humidity ( water evaporation rate) in Mombasa is higher than that of Nairobi.
- Since Mombasa has higher temperatures the rate of water evaporation is higher which results into a higer humidity. In return, this provides a favorable condition for rusting.
- Since Mombasa is adjacent to the Indian Ocean; the ocean salt, combines with the humidity and the winds, which results in natural corrosion on metals (Rust). Therefore cars are more likely to rust faster in Mombasa than Nairobi. Oxygen (O2) Properties and UsesOxygen (O2) is an active, life-sustaining component of the atmosphere; making up 20% by volume of the air we breathe.
It is colorless, odorless and tasteless.
Oxygen is the most widely occurring element on earth.
Because it forms compounds with virtually all chemical elements except the noble gases, most oxygen is bound with other elements in compounds such as silicates, oxides, and water.
It is also dissolved in rivers, lakes, and oceans.
Molecular oxygen occurs almost entirely in the atmosphere.
Oxygen is highly oxidizing (a general chemical term applying to any substance, like oxygen, that accepts electrons from another substance during reaction).
Oxygen reacts vigorously with combustible materials, especially in its pure state, generating heat in the reaction process.
Ozone (O3) is an allotropic form of oxygen that is more reactive than ordinary oxygen. Ozone is formed in nature by electrical discharges or by irradiation with ultraviolet light. Oxygen has a low boiling/ condensing point: -297.3°F (-183°C).
The gas is approximately 1.1 times heavier than air and is slightly soluble in water and alcohol. Below its boiling point, oxygen is a pale blue liquid slightly heavier than water.
Preparation of oxygen
Oxygen is prepared in the lab by catalytic decomposition of hydrogen peroxide using manganese (IV) oxideas shown. The gas is then collected over water.
Oxygen From the AirOxygen may be obtained from the atmosphere by the liquefaction and fractional distillation of air. Liquid air is a mixture of liquid nitrogen, boiling point -1960C, and liquid oxygen, boiling point -1830C.
The nitrogen is more volatile (i.e. it has a lower boiling point) and boils off first during evaporation. Because some oxygen evaporates with the nitrogen, separation of the two gases is brought about by fractionation (i.e. by letting the evolved gas mixture bubble through liquid air rich in oxygen in a tall rectifying column).
The oxygen in the gas mixture condenses and almost pure nitrogen gas leaves the top of the column, leaving almost pure liquid oxygen which is then evaporated to give oxygen gas.
The oxygen gas is distributed as a compressed gas in high pressure cylinders.
The process of fractional distillation involves essentially two stages.
a) First the air must be cooled until it turns into a liquid.
b) Then the liquid is allowed to warm up again. The various gases boil off at different temperatures.
Uses of oxygen1. Oxygen is used with fuel gases in gas welding and gas cutting
2. The largest user of oxygen is the steel industry.
3. Chemicals, Pharmaceuticals and Petroleum
4. Oxygen is increasingly important as a bleaching chemical
5. In medicine, oxygen is used during surgery, intensive care treatment, inhalation therapy, etc. High standards of purity and handling must be maintained.
6. Ozone is used for drinking water treatment, in particular when alternatives, such as chlorine, are undesirable.
Other uses
1. Oxygen has many uses in breathing apparatus, such as those for underwater work and refinery and chemical plant self contained breathing apparatus.
2. Aquaculture, the cultivation of fish in ponds uses oxygenated water to increase yields.
3. Liquid oxygen is used in liquid-fueled rockets as the oxidizer for liquid hydrogen and liquid methane.
The Reactivity Series
Reaction with Air (Oxygen).
Potassium, sodium, lithium, calcium and magnesium react with oxygen and burn in air. Metals in the reactivity series from aluminium to copper react with oxygen in the air to form the metal oxide.
Aluminium is the fastest and copper is the slowest of the six.
Aluminium reacts quickly to form a surface layer of aluminium oxide.
aluminium + oxygen aluminium oxide.
4Als + 3O2g 2Al2O3s/sub>
Zinc reacts fairly quickly to form zinc oxide.
zinc + oxygen zinc oxide.
2Zns + O2g 2ZnOs
Iron reacts slowly at room temperature but quickly if it is heated.
iron + oxygen iron(III) oxide – see rusting.
4Fes + 3O2g 2Fe2O3s/sub>
Metals below iron react with oxygen when they are heated in air.
tin + oxygen tin(II) oxide.
2Sn(s) + O2(g) 2SnO(g)
lead + oxygen lead(II) oxide.
2Pb(s) + O2(g) 2PbO(s)
copper + oxygen copper(II) oxide.
2Cu(s) + O2(g/sub>) 2CuO(s)
Silver, gold and platinum do not react with oxygen in the air.
Oxygen summary Physical properties
Footnotes:
- Makes up 20% of the gases in the air.
- Needed by the majority of living organisms for respiration
- Is produced by green plants as a by-product of photosynthesis. Summary of reactions of certain elements with oxygen
Water and HydrogenHydrogen is the simplest element. It is the first element in the periodic table, and it is placed in Group I of the periodic table.
Hydrogen
Occurrence
Hydrogen is the lightest element and the most abundant element in the universe.
Hydrogen occurs naturally as a mixture of the three isotopes:
- Protium, H,
- Deuterium, D, (which is also called Heavy Hydrogen) and
- Tritium, T. Preparation of Hydrogen by the Action of MetalsThe alkali metals, lithium, sodium, and potassium react violently with water at the ordinary temperature, yielding hydrogen.
2 Li + 2 H2O H2 + 2 LiOH
Calcium reacts with water more slowly unless the water is hot, when the action is more vigorous.
Ca + 2 H2O H2 + Ca(OH)2
Preparation of Hydrogen from Action of Acids
Hydrogen is prepared in the laboratory by the action of acids on metals.
Dilute sulphuric acid containing 1 volume of concentrated acid to 5 volumes of water, or dilute hydrochloric acid containing 1 volume of concentrated acid to 4 volumes of water, is added to granulated zinc.
Zinc sulphate or zinc chloride is formed in solution and the hydrogen that is evolved is collected over water in a trough.
Since hydrogen is very much lighter than air it may also be collected by upward displacement.Warning!
Before collecting hydrogen great care must be taken to ensure that all the air has been displaced from the apparatus since a mixture of hydrogen with air is highly explosive.
Properties
Hydrogen is;
• a colourless odourless gaseous element,
• sparingly soluble in water and the solubility is not much affected by change of temperature,
• Does not support respiration although it is not poisonous. When hydrogen is breathed mixed with some air for a short time, it weakens the voice and raises its pitch,
• a better conductor of heat than other gases, its conductivity being about five times that of air, and
• Forms compounds with a large number of elements. In many cases, these compounds are formed by the direct combination of the elements.
• Chemically, hydrogen reacts with most elements.
Hydrogen summary
Physical Properties
Footnotes:
- The lightest gas known.
- Once used in airships but replaced by helium which is not explosive.
- Used to make ammonia which is needed in the manufacture of fertilizers and explosives.Water on Earth
Water is an important item in out universe. We need water for transport (lakes and oceans), Generation of power, (hydroelectricity), drinking industrial processes, manufacturing and cooling among others.
Very little of the world’s water is fresh (2.6%).
Most of it (97.4%) is in oceans.
Most of the fresh water is frozen (76%). Only a tiny fraction is available for human use, about 0.01%.
Combustion of HydrogenHydrogen burns in oxygen or air to form water.
2 H2 + O2 2 H2 O
Oxygen will also burn in hydrogen. Hydrogen does not itself support combustion, as may be shown by passing a lighted taper into an inverted jar of hydrogen, when the taper is extinguished.
A mixture of hydrogen with oxygen or air explodes violently when kindled, provided either gas is not present in too large excess.
Reaction with Non-Metals
Hydrogen readily combines with fluorine and chlorine, less readily with bromine, iodine, sulphur, phosphorous, nitrogen, and carbon.
H2 + F2 2 HF
Hydrogen burns in chlorine gas and a mixture of hydrogen and chlorine explodes violently when kindled or exposed to bright sunlight.
H2 + Cl2 2 HCl
Hydrogen combines with nitrogen on sparking or in presence of a catalyst, forming ammonia.
N2 + 3 H2 2 NH2
Reducing Properties
When hydrogen is passed over many heated metallic oxides (e.g. copper oxide, iron oxide, or lead oxide), they are reduced to the metals.
In this arrangement, dry hydrogen is passed over heated copper (II) oxide.
The copper oxide is reduced to red brown copper metal, while hydrogen oxidizes to water.
UsesHydrogen is used;
• in the reduction of oxide ores,
• in the refining of petroleum,
• in the production of hydrocarbons from coal,
• to fill balloons and airships, as it is the least dense gas known (i.e. it is lighter than air).
Previously, coal gas was often used for the same purpose, as it contains a high percentage of hydrogen.
However, because the flammable nature of hydrogen makes it dangerous for such use, this use of hydrogen has been to replaced by helium,
• in the Synthesis of ammonia,
• as a fuel in Oxy-Hydrogen blowpipes,
• for the hardening of vegetable or animal oils (i.e. to convert them into saturated fats which are solids), and
• for the hydrogenating petroleum fractions, coal and other organic compounds.
Principal Compounds of hydrogen
Hydrogen is widely distributed in industrially important compounds and is present
1. In a wide range of inorganic compounds, including
- Ammonia
- Hydrogen Sulphide
- Water2. In the strong acids, including
- Sulphuric Acid
- Nitric Acid
- Hydrochloric Acid
- Hydrobromic Acid
- Hydrofluoric Acid3. In almost all organic compounds.
- Alkanes
- Alkenes
- AlkynesAlcohols
Answers
Activity 1
1. Beakers and conical flasks
2. Measuring cylinder
3. Using a balance
4. Goggles and fume hood
KCSE Form 1 Chemistry Notes
Introduction to chemistryChemistry is a branch of Science. Science is basically the study of living and non-living things.
The branch of science that study living things is called Biology.The branch of science that study non-living things is called Physical Science.Physical Science is made up of:
(i)Physics– the study of matter in relation to energy
(ii)Chemistry– the study of composition of matter.
Chemistry is thus defined as the branch of science that deals with the structure composition, properties and behavior of matter.
Basic Chemistry involves studying:
(a)States/phases of matter
Matter is anything that has weight/mass and occupies space/volume.Naturally, there are basically three states of matter.
(i) Solid-e.g. soil, sand, copper metal ,bucket, ice.
(ii)Liquid-e.g water, Petrol, ethanol/alcohol, Mercury(liquid metal).
(iii)gas- e.g. Oxygen, Nitrogen ,Water vapour.
A solid is made up of particles which are very closely packed. It thus has a definite/fixed shape and fixed/definite volume/occupies definite space. It has a very high density.
A liquid is made up of particles which have some degree of freedom.
It thus has no definite/fixed shape.It takes the shape of the container it is put.
A liquid has fixed/definite volume/occupies definite space.
A gas is made up of particles free from each other.
It thus has no definite/fixed shape. It takes the shape of the container it is put.
It has no fixed/definite volume/occupies every space in a container.
(b) Separation of mixture
A mixture is a combination of two or more substances that can be separated by physical means.
Simple methods of separating mixtures at basic chemistry level include
(i)Sorting/picking-this involve physically picking one pure substance from a mixture with another/other. e. g. sorting maize from maize beans mixture.
(ii)Decantation-this involve pouring out a liquid from a solid that has settled/sinking solid in it. e. g. Decanting water form sand.
(iii)Filtration-this involves sieving /passing particles of a mixture through a filter containing small holes that allow smaller particle to pass through but do not allow bigger particle to pass through.
(iv)Skimming-this involve scooping floating particles. e.g. cream from milk
(c) Metals and non-metals
Metals are shiny,ductile(able to form wires),malleable(able to form sheet) and coil without breaking. e.g. Iron, gold, silver, copper. Mercury is the only liquid metal known.
Non-metals are dull, not ductile(do not form wires), not malleable(do not form sheet) and break on coiling/brittle. e.g. Charcoal, Sulphur , plastics.
(d)Conductors and non-conductors
A conductor is a solid that allow electric current to pass through.
A non-conductor is a solid that do not allow electric current to pass through.
All metals conduct electricity. All non-metals do not conduct electricity except carbon graphite.
(e)Drugs
A drug is a natural or synthetic/man-made substance that when taken changes/alter the body functioning.
A natural or synthetic/man-made substance that when taken changes/alter the abnormal body functioning to normal is called medicine.
Medicines are thus drugs intended to correct abnormal body functions.. Medicines should therefore be taken on prescription and dosage.
A prescription is a medical instruction to a patient/sick on the correct type of medicine to take and period/time between one intake to the other .
A dosage is the correct quantity of drug required to alter the abnormal body function back to normal. This is called treatment.
It is the professional work of qualified doctors/pharmacists to administer correct prescription and dosage of drugs/medicine to the sick.
Prescription and dosage of drugs/medicine to the sick use medical language.
Example
(i) 2 x 4; means “2” tablets for solid drugs/spoon fulls for liquid drugs taken “4” times for a duration of one day/24 hours and then repeated and continued until all the drug given is finished.
(ii) 1 x 2; means “1” tablets for solid drugs/spoon fulls for liquid drugs taken “2” times for a duration of one day/24 hours and then repeated and continued until all the drug given is finished.
Some drugs need minimal prescription and thus are available without pharmacist/ doctor’s prescription.
They are called Over The Counter(OTC) drugs.
OTC drugs used to treat mild headaches, stomach upsets, common cold include:
(i) painkillers
(ii) anti acids
(iii) cold/flu drugs.
All medicines require correct intake dosage. When a prescription dosage is not followed,this is called drug misuse/abuse.
Some drugs are used for other purposes other than that intended.
This is called drug abuse. Drug abuse is when a drug is intentionally used to alter the normal functioning of the body.
The intentional abnormal function of the drug is to make the victim have false feeling of well being.
The victim lack both mental and physical coordination.
Some drugs that induce a false feeling of well being are illegal. They include heroin, cocaine, bhang, mandrax and morphine.
Some abused drugs which are not illegal include: miraa, alcohol, tobacco, sleeping pills.
The role of chemistry in society
(a)Chemistry is used in the following:
(i)Washing/cleaning with soap:
Washing/cleaning is a chemical process that involve jnteraction of water,soap and dirt so as to remove the dirt from a garment.
(ii)Understanding chemicals of life
Living thing grow, respire and feed. The formation and growth of cells involve chemical processes in living things using carbohydrates, proteins and vitamins.
(iii)Baking:
Adding baking powder to dough and then heating in an oven involves interactions that require understanding of chemistry.
(iv)Medicine:
Discovery,test ,prescription and dosage of drugs to be used for medicinal purposes require advanced understanding of chemistry
(v)Fractional distillation of crude oil:
Crude oil is fractional distilled to useful portions like petrol,diesel,kerosene by applying chemistry.
(vi)Manufacture of synthetic compounds/substances
Large amounts of plastics,glass,fertilizers, insecticides, soaps, cements, are manufactured worldwide.
Advanced understanding of the chemical processes involved is a requirement.
(vii)Diagnosis/test for abnormal body functions.
If the body is not functioning normally,it is said to be sick/ill.Laboaratory test are done to diagnose the illness/sickness.
(b)The following career fields require Chemistry as one of subject areas of advanced/specialized study:
(i)Chemical engineering/chemical engineer
(ii)Veterinary medicine/Veterinary doctor
(iii)Medicine/Medical doctor/pharmacist/nurse
(iv)Beauty/Beautician
(v)Teaching/Chemistry teacher.
The School Chemistry Laboratory
Chemistry is studied mainly in a science room called a school chemistry laboratory.
The room is better ventilated than normal classroom. It has electricity, gas and water taps.
Aschool chemistry laboratory has a qualified professional whose called Laboratory technician/assistant.
All students user in a school chemistry laboratory must consult the Laboratory technician/assistant for all their laboratory work.
A school chemistry laboratory has chemicals and apparatus.
A chemical is a substance whose composition is known. All chemical are thus labeled as they are.
This is because whereas physically a substance may appear similar, chemically they may be different.
All Chemicals which are not labeled should never be use.
Some chemicals are toxic/poisonous, explosive, corrosive, caustic, irritants, flammable, oxidizing, carcinogenic, or radioactive.
Care should always be taken when handling any chemical which have any of the above characteristic properties.
Common school chemistry laboratory chemicals include:
(i)distilled water
(ii)Concentrated mineral acid which are very corrosive(on contact with skin they cause painful open wounds)
(iii)Concentrated alkali/bases which are caustic(on contact with skin they cause painful blisters)
(iv)Very many types of salts
The following safety guideline rules should be followed by chemistry laboratory users:
(i)Enter the laboratory with permission in an orderly manner without rushing/pushing/scrabbling.
(ii)Do not try unauthorized experiments. They may produce flammable, explosive or toxic substances that affect your health.
(iii)Do not taste any chemical in the laboratory. They may be poisonous.
(iv)Waft gas fumes to your nose with your palm.Do not inhale/smell gases directly. They may be highly poisonous/toxic.
(v)Boil substances with mouth of the test tube facing away from others and yourself.
Boiling liquids spurt out portions of the hot liquid. Products of heating solids may be a highly poisonous/toxic gas.
(vi)Wash with lots of water any skin contact with chemicals immediately.
Report immediately to teacher/laboratory technician any irritation,cut,burn, bruise or feelings arising from laboratory work.
(vii)Read and follow safety instruction.All experiments that evolve/produce poisonous gases should be done in the open or in a fume chamber.
(viii)Clean your laboratory work station after use.Wash your hand before leaving the chemistry laboratory.
(ix)In case of fire, remain calm, switch of the source of fuel-gas tap. Leave the laboratory through the emergency door. Use fire extinguishers near the chemistry laboratory to put of medium fires. Leave strong fires wholly to professional fire fighters.
(x)Do not carry unauthorized item from a chemistry laboratory.
An apparator /apparatus are scientific tools/equipment used in performing scientific experiments.
The conventional apparator used in performing a scientific experiments is called standard apparator/apparatus.
If the conventional standard apparator/apparatus is not available, an improvised apparator/apparatus may be used in performing a scientific experiments.
An improvised apparator/apparatus is one used in performing a scientific experiment for a standardapparator/apparatus.
Most standard apparatus in a school chemistry laboratory are made of glass because:
(i)Glass is transparent and thus reactions /interactions inside are clearly visible from outside
(ii)Glass is comparatively cheaperwhich reduces cost of equipping theschool chemistry laboratory
(iii)glass is comparatively easy to clean/wash after use.
(iv)glass is comparatively unreactive to many chemicals.
Apparatus are designed for the purpose they are intended in a school chemistry laboratory:
(a)Apparatus for measuring volume
1. Measuring cylinder
Measuring cylinders are apparatus used to measure volume of liquid/ solutions.
They are calibrated/graduated to measure any volume required to the maximum. Measuring cylinders are named according to the maximum calibrated/graduated volume e.g.
“10ml” measuring cylinder is can hold maximum calibrated/graduated volume of “10mililitres” /“10 cubic centimetres”
“50ml” measuring cylinder is can hold maximum calibrated/graduated volume of “50mililitres” /“50 cubic centimetres”
“250ml” measuring cylinder is can hold maximum calibrated/graduated volume of “250mililitres” /“250 cubic centimetres”
“1000ml” measuring cylinder is can hold maximum calibrated/graduated volume of “1000mililitres” /“1000 cubic centimetres”
2.Burette
Burette is a long and narrow/thin apparatus used to measure small accurate and exact volumes of a liquid solution.
It must be clamped first on a stand before being used.
It has a tap to run out the required amount out.
They are calibrated/graduated to run out small volume required to the maximum 50ml/50cm3.
The maximum 50ml/50cm3 calibration/graduation reading is at the bottom .This ensure the amount run out from a tap below can be determined directly from burette reading before and after during volumetric analysis.
Burettes are expensive and care should be taken when using them.
3.(i) Pipette
Pipette is a long and narrow/thin apparatus that widens at the middle used to measure and transfer small very accurate/exact volumes of a liquid solution.
It is open on either ends.
The maximum 25ml/25cm3 calibration/graduation mark is a visible ring on one thin end. To fill a pipette to this mark, the user must suck up a liquid solution upto a level above the mark then adjust to the mark using a finger.
This require practice.
(ii)Pipette filler
Pipette filler is used to suck in a liquid solution into a pipette instead of using the mouth.
It has a suck, adjust and eject button for ensuring the exact volume is attained.
This requires practice.
4.Volumetric flask.
A volumetric flask is thin /narrow but widens at the base/bottom.
It is used to measure very accurate/exact volumes of a liquid solution.
The maximum calibration /graduation mark is a visible ring.
Volumetric flasks are named according to the maximum calibrated/graduated volume e.g. “250ml” volumetric flask has a calibrated/graduated mark at exact volume of “250mililitres” /“250centimetres”
“1l” volumetric flask has a calibrated/graduated mark at exact volume of “one litre” /“1000 cubic centimetres”
“2l” volumetric flask has a calibrated/graduated mark at exact volume of “two litres” /“2000 cubic centimetres”
5. Dropper/teat pipette
A dropper/teat pipette is a long thin/narrow glass/rubber apparatus that has a flexible rubber head. A dropper/teat pipette is used to measure very small amount/drops of liquid solution by pressing the flexible rubber head.
The number of drops needed are counted by pressing the rubber gently at a time .
(b)Apparatus for measuring mass
1.Beam balance
A beam balance has a pan where a substance of unknown mass is placed. The scales on the opposite end are adjusted to “balance” with the mass of the unknown substance.
The mass from a beam balance is in grams.
2.Electronic/electric balance.
An electronic/electric balance has a pan where a substance of unknown mass is placed.The mass of the unknown substance in gramsis available immediately on the screen.
(c)Apparatus for measuring temperature
A thermometer has alcohol or mercury trapped in a bulb with a thin enclosed outlet for the alcohol/mercury in the bulb.
If temperature rises in the bulb, the alchohol /mercury expand along the thin narrow enclosed outlet.
The higher the temperature,the more the expansion.
Outside, a calibration /graduation correspond to this expansion and thus changes in temperature.
A thermometer therefore determines the temperature when the bulb is fully dipped in to the substance being tested. To determine the temperature of solid is thus very difficult.
(d)Apparatus for measuring time
The stop watch/clock is the standard apparatus for measuring time.Time is measured using hours, minutes and second.
Common school stop watch/clock has start, stop and reset button for determining time for a chemical reaction.This require practice.
(e) Apparatus for scooping
1. Spatula
A spatula is used to scoop solids which do not require accurate measurement. Both ends of the spatula can be used at a time.
A solid scooped to the brim is “one spatula end full”A solid scooped to halfbrim is “half spatula end full”.
2. Deflagrating spoon<> A deflagrating spoon is used to scoop solids which do not require accurate measurement mainly for heating. Unlike a spatula, a deflagrating spoon is longer.
(f) Apparatus for putting liquids/solid for heating.
1.Test tube.
A test tube is a narrow/thin glass apparatus open on one side. The end of the opening is commonly called the “the mouth of the test tube”.
2. Boiling/ignition tube.
A boiling/ignition tube is a wide glass apparatus than a test tube open on one side.
The end of the opening is commonly called the “the mouth of the boiling/ignition tube”.
3. Beaker.
Beaker is a wide calibrated/graduated lipped glass/plastic apparatus used for transferring liquid solution which do not normally require very accurate measurements
Beakers are named according to the maximum calibrated/graduated volume they can hold e.g. “250ml” beaker has a maximum calibrated/graduated volume of “250mililitres” /“250 cubic centimetres”
“1l” beaker has a maximum calibrated/graduated volume of “one litre” /“1000 cubic centimetres”
“5 l” beaker has a maximum calibrated/graduated volume of “two litres” /“2000 cubic centimetres”
4. Conical flask.
A conical flask is a moderately narrow glass apparatus with a wide base and no calibration/graduation. Conical flasks thus carry/holdexact volumes of liquids that have been measured using other apparatus.
It can also be put some solids. The narrow mouth ensures no spirage.
Conical flasksare named according to the maximum volume they can hold e.g. “250ml” Conical flasks hold a maximum volume of “250mililitres” /“250 cubic centimetres” “500ml” Conical flasks hold a maximum volume of “500ml” /“1000 cubic centimetres”
5. Round bottomed flask
A round bottomed flask is a moderately narrow glass apparatus with a wide round base and no calibration/graduation.
Round bottomed flask thus carry/hold exact volumes of liquids that have been measured using other apparatus.
The narrow/thin mouth prevents spirage. The flask can also hold (weighed) solids.
A round bottomed flask must be held/ clamped when in use because of its wide narrow base.
6. Flat bottomed flask
A flat bottomed flask is a moderately narrow glass apparatus with a wide round base with a small flat bottom.
It has no calibration/graduation.
Flat bottomed flask thus carry/hold exact volumes of liquids that have been measured using other apparatus.
The narrow/thin mouth prevents spirage. They can also hold (weighed) solids.
A flat bottomed flask must be held/ clamped when in use because it’s flat narrow base is not stable.
(g) Apparatus for holding unstable apparatus( during heating).
1.Tripod stand
A tripod stand is a three legged metallic apparatus which unstable apparatus are placed on (during heating).
Beakers. conical flasks,round bottomed flask and flat bottomed flasks are placed on top of tripod stand (during heating).
2.Wire gauze/mesh
Wire gauze/mesh is a metallic/iron plate of wires crossings. It is placed on top of a tripod stand:
(i) ensure even distribution of heat to prevent cracking glass apparatus
(ii) hold smaller apparatus that cannot reach the edges of tripod stand
3 Clamp stand
A clamp stand is a metallic apparatus which tightly hold apparatus at their “neck” firmly.
A clamp stand has a wide metallic base that ensures maximum stability.
The heightand position of clamping is variable. This require practice
4.Test tube holder
A test tube holder is a hand held metallic apparatus which tightly hold test/boiling/ignition tube at their “neck” firmly on the other end.
Some test tube holders have wooden handle that prevent heat conduction to the hand during heating.
5. Pair of tong.
A pair of tong is a scissor-like hand held metallic apparatus which tightly hold firmly a small solid sample on the other end.
6.Gas jar
A gas jar is a long wide glass apparatus with a wide base.
It is open on one end. It is used to collect/put gases.
This requires practice.
(h) Apparatus for holding/directing liquid solutions/funnels ( to avoid spirage).
1.Filter funnel
A filter funnel is a wide mouthed (mainly plastic) apparatus that narrow drastically at the bottom to a long extension.
When the long extension is placed on top of another apparatus, a liquid solution can safely be directed through the wide mouth of the filter funnel into the apparatus without spirage.
Filter funnel is also used to place a filter paper during filtration.
2.Thistle funnel
A thistle funnel is a wide mouthed glass apparatus that narrow drastically at the bottom to a very long extension.
The long extension is usually drilled through a stopper/cork.
A liquid solution can thus be directed into a stoppered container without spirage
3. Dropping funnel
A dropping funnel is a wide mouthed glass apparatus with a tap that narrow drastically at the bottom to a very long extension.
The long extension is usually drilled through a stopper/cork.
A liquid solution can thus be directed into a stoppered container without spirage at the rate determined by adjusting the tap.
4. Separating funnel
A separating funnel is a wide mouthed glass apparatus with a tap at the bottom narrow extension.
A liquid solution can thus be directed into a separating funnel without spirage. It can also safely be removed from the funnel by opening the tap.
It is used to separate two or more liquid solution mixtures that form layers/immiscibles. This requires practice.
(h) Apparatus for heating/Burners
1. Candle, spirit burner, kerosene stove, charcoal burner/jiko
are some apparatus that can be used for heating.
Any flammable fuel when put in a container and ignited can produce some heat.
2.Bunsen burner
The Bunsen burner is the standard apparatus for heating in a Chemistry school laboratory. It was discovered by the German Scientist Robert Wilhelm Bunsen in 1854.
(a)Diagram of a Bunsen burner
A Bunsen burner uses butane/laboratory gas as the fuel.The butane/laboratory gas is highly flammable and thus usually stored safely in a secure chamber outside Chemistry school laboratory.
It is tapped and distributed into the laboratory through gas pipes.
The gas pipes end at the gas tap on a chemistry laboratory bench .If opened the gas tap releases butane/laboratory gas.
Butane/laboratory gas has a characteristic odour/smell that alerts leakages/open gas tap.
The Bunsen burner is fixed to the gas tap using a strong rubber tube.
The Bunsen burner is made up of the following parts:
(i)base plate –to ensure the burner can stand on its own
(ii)Jet-a hole through which laboratory gas enters the burner
(iii)Collar/sleeve-adjustable circular metal attached to the main chimney/burell with a side hole/entry. It controls the amount of air entering used during burning.
(iv)Air hole- a hole/entry formed when the collar side hole is in line with chimney side hole.
If the collar side hole is not in line with chimney side hole, the air hole is said to be “closed”If the collar side hole is in line with chimney side hole, the air hole is said to be “open”
(v)Chimney- tall round metallic rod attached to the base plate.
(b)Procedure for lighting/igniting a Bunsen burner
1. Adjust the collar to ensure the air holes are closed.
2. Connect the burner to the gas tap using a rubber tubing. Ensure the rubber tubing has no side leaks.
3. Turn on the gas tap.
4. Ignite the top of the chimney using a lighted match stick/gas lighter/wooden splint.
5.Do not delay excessively procedure (iv) from (iii) to prevent highly flammable laboratory gas from escaping/leaking.
(c)Bunsen burner flames
A Bunsen burner produces two types of flames depending on the amount of air entering through the air holes.
If the air holes are fully open, a non luminous flame is produced. If the air holes are fully closed, a luminous flame is produced.
If the air air holes are partially open/ closed, a hybrid of non luminous and luminous flames is produced.
Luminous flame has three main regions:(i)the top yellow region where there is incomplete combustion/burning
(ii)the region of unburnt gas below the yellow region where the gas does not burn
(iii) blue region on the sides of region of unburnt gas where there is complete burning
Non-luminous flame has four main regions:
(i)the top colourless region
(ii) blue region just below where there is complete burning.It is the hottest region
(iii) green region surrounded by the blue region where there is complete burning
(ii)the region of unburnt gas at the innermost surrounded by green and blue regions. No burning takes place here
Scientific apparatus are drawn:
(i)using a proportional two dimension(2D) cross-sections. Three dimensions (3D) are not recommended.
(ii)straight edges of the apparatus on a scientific diagram should be drawn using ruler.
(iii)curved edges of the apparatus on a scientific diagram should be drawn using free hand.
(iv)The bench, tripod or clamp to support apparatus which cannot stand on their own should be shown.
Classification of Substances
Substances are either pure or impure. A pure substance is one which contains only one substance.
An impure substance is one which contains two or more substances. A pure substance is made up of a pure solid, pure liquid or pure gas.
A mixture is a combination of two or more pure substances which can be separated by physical means.The three states of matter in nature appear mainly as mixtures of one with the other.
Common mixtures include:
(a)Solutions/solid-liquid dissolved mixture
Experiment:
To make a solution of copper(II)sulphate(VI)/Potassium manganate(VII)/sodium chloride
Procedure
Put about 100 cm3 of water in three separate beakers. Separately place a half spatula end full of copper(II)sulphate(VI) ,Potassium manganate(VII) and sodium chloride crystals to each beaker. Stir for about two minutes.
Observation
Copper(II)sulphate(VI) crystals dissolve to form a blue solution
Potassium manganate(VII) crystals dissolve to form a purple solution
Sodium chloride crystals dissolve to form a colourless solution
Explanation
Some solids, liquids and gases dissolve in some other liquids.
A substance/liquid in which another substance dissolves is called solvent.
A substance /solid /gas which dissolves in a solvent is called solute.
When a solute dissolves in a solvent it forms a uniform mixture called solution.
A solute dissolved in water as the solvent exists in another state of matter called aqueous state.Water is refered as the universal solvent because it dissolves many solutes.
A solute that dissolves in a solvent is said to be soluble.Soluble particles uniformly spread between the particles of water/solvent and cannot be seen.
Solute + Solvent -> solution
Solute + Water -> Aqueous solution of solute
The solute dissolved in water gives the name of the solution
e. g. 1. Sodium chloride solution is a solution formed after dissolving sodium chloride
crystals/solid in water.Sodium chloride exists in aqueous state after dissolving.
Sodium chloride + Water -> Sodium chloride solution
NaCl(s) + (aq) -> NaCl(aq)
2. Ammonia solution is a solution formed after dissolving ammonia gas in water. Ammoniaexists in aqueous state after dissolving.
Ammonia gas + Water -> Aqueous ammonia
NH3(g) + (aq) -> NH3(aq)
3.Copper(II)sulphate(VI) solution is a solution formed after dissolving Copper(II) sulphate(VI) crystals/solid in water.Copper(II)sulphate(VI) exist in aqueous state after dissolving.
Copper (II)sulphate(VI) + Water -> Copper (II)sulphate(VI) solution
CuSO4(s) + (aq) -> CuSO4 (aq)
4.Potassium manganate(VII) solution is a solution formed after dissolving Potassium manganate(VII) crystals/solid in water.
Potassium manganate(VII)exist in aqueous state after dissolving.
Potassium manganate(VII) + Water ->Potassium manganate(VII) solution
KMnO4(s) +(aq) -> KMnO4 (aq)
(b)Suspension/ precipitates/solid-liquid mixture which do not dissolve
Experiment: To make soil,flour and Lead(II)Iodide suspension/precipitate
Procedure
Put about 100 cm3 of water in three separate beakers. Separately place a half spatula end full of soil ,maize and lead(II)Iodide to each beaker. Stir for about two minutes.
Observation
Some soil , maize and lead(II)Iodide float in the water
A brown suspension/precipitate/particles suspended in water containing soil
A white suspension/precipitate/particles suspended in water containing flour
A yellow suspension/precipitate/particles suspended in water containing Lead(II)iodide.
Some soil , maize and lead(II)Iodide settle at the bottom after some time.
Explanation
Some solid substances do not dissolve in a liquid.
They are said to be insoluble in the solvent .
When an insoluble solid is put in liquid:
(i) some particles remain suspended/floatingin the liquid to form a suspension/precipitate.
(ii) some particles sink/settle to the bottom to form sediments after being allowed to stand .
An insoluble solid acquire the colour of the suspension/precipitate .e.g .
1.A white suspension /precipitate has some fine white particles suspended/floating in the liquid.Not “white solution”
2.A blue suspension /precipitate has some fine blue particles suspended/floating in the liquid.
3.A green suspension /precipitate has some fine green particles suspended/floating in the liquid.
4.A brown suspension /precipitate has some fine brown particles suspended/floating in the liquid.
4.A yellow suspension /precipitate has some fine yellow particles suspended/floating in the liquid.
(c) (i) Miscibles/Liquid-liquid mixtures
To form water-ethanol and Kerosene-turpentine miscibles
Procedure
(i)Measure 50cm3 of ethanol into 100cm3 beaker. Measure 50cm3 of water. Place the water into the beaker containing ethanol. Swirl for about one minute.
(ii)Measure 50cm3 of kerosene into 100cm3 beaker. Measure 50cm3 of turpentine oil. Place the turpentine oil into the beaker containing kerosene. Swirl for about one minute.
Observation
Two liquids do not form layers.
Ethanol and water form a uniform mixture.
Kerosene and turpentine oil form uniform mixture
Explanation
Ethanol is miscible in Water.Kerosene is miscible in turpentine oil.Miscible mixture form uniform mixture. They do not form layers. The particles of one liquid are smaller than the particles of the other.
The smaller particles occupy the spaces between the bigger particles.
(ii) Immiscibles /Liquid-liquid mixtures
To form water-turpentine oiland Kerosene-water miscibles
Procedure
(i)Measure 50cm3 of water into 100cm3 beaker. Measure 50cm3 of turpentine oil. Place the oil into the beaker containing water. Swirl for about one minute.
(ii)Measure 50cm3 of water into 100cm3 beaker. Measure 50cm3 of kerosene. Place the kerosene into the beaker containing water. Swirl for about one minute.
Observation
Two liquids form layers.
Turpentine and water do not form a uniform mixture.
Water and kerosene do not form uniform mixture
Explanation
Kerosene is immiscible in Water.Water is immiscible in turpentine oil. Immiscible mixtures do not form uniform mixtures.
They form layers. The size of the particles of one liquid isalmost equal to the particles of the other.
The particles of one liquid cannot occupy the spaces between the particles of the other.
The heavier particles settle at the bottom. The less dense particles settle on top.
(d)Solid-solid mixtures/Alloys
Before solidifying, some heated molten/liquid metals dissolve in another metal to form a uniform mixture of the two.
On solidifying, a uniform mixture of the metals is formed.
A uniform mixture of two metals on solidifying is called alloy.
In the alloy, one metallic particle occupies the spaces between the metallic particles of the other.
c) Common alloys of metal.
Methods of Separating MixturesMixtures can be separated from applying the following methods:
(a) Decantation
Sediments can be separated from a liquid by pouring out the liquid. This process is called decantation.
Experiment
Put some sand in a beaker. Add about 200cm3 of water. Allow sand to settle. Pour off water carefully into another beaker.
Observation
Sand settles at the bottom as sediments.
Less clean water is poured out.
Explanation
Sand does not dissolve in water. Sand is denser than water and thus settles at the bottom as sediment. When poured out, the less dense water flows out.
(b)Filtration
Decantation leaves suspended particles in the liquid after separation. Filtration is thus improved decantation.
Filtration is the method of separating insoluble mixtures/particles/solids from a liquid.
Experiment:
To separate soil and water using filtration
Fold a filter paper to fit well into a filter funnel. Place the funnel in an empty 250cm3 beaker.
Put one spatula end full of soil into 50cm3 of water. Stir. Put the soil/water mixture into the filter funnel.
Observations
Clean water is collected below the filter funnel.
Soil remains above the filter paper.
Explanation
A filter paper is porous which act like a fine sieve with very small holes.
The holes allow smaller water particles to pass through but do not allow bigger soil particles.
The liquid which passes through is called filtrate. The solid which do not pass through is called residue.
Set up of apparatus
(c)EvaporationEvaporation is a method of separatinga solute/solid from its solution.
This involves heating a solution(solvent and solute)to vapourize the solvent out of the solution mixture leaving pure solute/solid.
If a mixture contain insoluble solid,they are filtered out.
Experiment: To separate a mixture of soil and salt(sodium chloride) .
Procedure:
Put one spatula end full of soil on a filter paper.
Put one spatula full of common salt/sodium chloride into the same filter paper. Mix well using the spatula,.
Place about 200cm3 of water into a beaker.
Put the contents of the filter paper into the water. Stir thoroughly using a glass/stirring rod for about one minute.
Fold a filter paper into a filter funnel.
Pour half portion of the contents in the beaker into the filter funnel.
Put the filtrate into an evaporating dish. Heat on a water bath.
Observation
(i)On mixing
Colourless crystals and brown soil particles appear on the filter paper.
(ii)On adding water
Common soil dissolves in water. Soil particles do not dissolve in water.
(iii)On filtration
Colourless liquid collected as filtrate below the filter funnel/paper.
Brown residue collected above the filter funnel/paper.
(iv)On evaporation
Colourless crystals crystals collected after evaporation
Explanation
Solid mixture of sand and common salt take the colours of the two.
On adding water,common salt dissolve to form a solution .
Soil does not because it is insoluble in water and thus forms a suspension.
On filtration, a residue of insoluble soil does not pass through the filter paper.
It is collected as residue.
Common salt solution is collected as filtrate.
On heating the filtrate, the solvent/water evaporate/vapourize out of the evaporating dish leaving common salt crystals.
Vapourization/evaporation can take place even without heating.
This is the principle/process of drying wet clothes on the hanging line.
Set up of apparatus
(d) DistillationDistillation is an improved evaporation where both the solute and the solvent in the solution are separated /collected.
Distillation therefore is the process of separating a solution into constituent solid solute and the solvent.
It involves heating the solution to evaporate/vapourize the solvent out. The solvent vapour is then condensed back to a liquid.
Experiment: To obtain copper(II)sulphate(VI) crystals and water from copper(II)sulphate(VI) solution.
Procedure:
Put one spatula end full of copper(II)sulphate (VI) crystals into a 250cm3 beaker. Place about 200cm3 of water into the beaker.
Stir thoroughly using a glass/stirring rod for about one minute.
Pour half portion of the contents in the beaker into a round bottomed/flat/conical flask broken porcelain/sand/glass into the flask.
Put a few pieces of b Stopper the flask.
Connect the flask to a liebig condenser using delivery tube.
Place a 200cm3 clean empty beaker/conical flask as a receiver at the end of the liebig condenser.
Circulate water in the liebig condenser.
Heat the flask strongly on a tripod stand with wire mesh/gauze until there is no more visible boiling bubbles in the flask.
Observation
Copper(II)sulphate (VI) crystals dissolve in water to form a blue solution.
On heating, colourless liquid is collected in the receiver.
Blue crystals are left in the flask.
(if gently heated further, the blue crystals turn to white powder)
Explanation
On heating blue Copper(II)sulphate (VI) solution, the colourless liquid solvent evaporate/vapourize .
The liquid vapour/gas passes through the delivery tube to the liebig condenser.
Theliebig condenser has a cold water inlet near the receiver and cold water out let.
This ensures efficient cooling. If thecold water outlet/inlet is reversed, the water circulation would be less efficient.
The water in the receiver would be warm.In the liebig condenser, the cold water,condenses the liquid vapour into liquid.
The condensed liquid collects in the receiver as distillate.
The solute of blue Copper(II)sulphate (VI) crystals is left in the flask as residue.
During simple distillation,therefore, the solution is heated to vapourize /evaporate the solvent/one component which is condensed at a different part of the apparatus.
The purpose of pieces of broken porcelain/porous pot/glass/sand/ is to:
(i)prevent bumping of the solution during boiling.
(ii)ensure smooth and even boiling.
Salty sea water can be made pure through simple distillation.
Any mixture with a large difference /40oC in boiling point can be separated using simple distillation.
Set up of apparatus
(e)Fractional distillationFractional distillation is an improved simple distillation used specifically to separate miscible mixtures with very close /near boiling points.
Fractional distillation involves:
(i)Heating the mixture in a conical/roundbottomed /flat bottomed flask.
The pure substance with a lower boiling point and thus more volatile evaporates/boils/vapourizes first. e.g.
Pure ethanol has a boiling point of 78oC.Pure water has a boiling point of 100oC at sea level/one atmosphere pressure.
When a miscible mixture of ethanol and water is heated,ethanol vapourizes/boils/evaporates first because it is more volatile.
(ii)The conical/round bottomed /flat bottomed flask is connected to a long glass tube called fractionating column.
The purpose of the fractionating column is to offer areas of condensation for the less volatile pure mixture.
The fractionating column is packed with glass beads/broken glass/porcelain/shelves to increase the surface area of condensation of the less volatile pure mixture.
(iii)When the vapours rise they condense on theglass beads/broken glass /porcelain / shelves which become hot.
When the temperature of the glass beads/broken glass/porcelain/shelves is beyond the boiling point of the less volatile pure substance, the pure substance rise and condensation take place on the glass beads/broken glass/porcelain/shelves at a higher level on the fractionating column.
The less volatile pure substance trickles/drips back down the fractionating column or back into theconical/round bottomed /flat bottomed flask to be heated again. e.g.
If the temperature on glass beads/broken glass/porcelain/shelves is beyond 78oC,the more volatile pure ethanol rise to condense on the glass beads/broken glass/porcelain/shelves higher in the fractionating column.
Water condenses and then drip/trickle tothe glass beads/broken glass /porcelain /shelves lower in the fractionating column because it is less volatile.
(iv)The fractionating column is connected to a liebig condenser.
The liebig condenser has a cold water inlet and outlet circulation.
The more volatile mixture that reach the top of the fractionating column is condenses by the liebig condenser into a receiver.
It is collected as the first fraction.
(v)At the top of the fractionating column, a thermometer is placed to note/monitor the temperature of the boiling mixtures .
Pure substances have constant/fixed boiling point. When one mixture is completely separated, the thermometer reading rises.
e.g. The thermometer reading remains at78oC when ethanol is being separated. When no more ethanol is being separated, the mercury/alcohol level in the thermometer rises.
(vi)The second /subsequent fractions are collected in the receiver after noting a rise the mercury/alcohol level in the thermometer. e.g.
The thermometer reading rises to 100oC when water is being separated.
It is passed through the liebig condenser with the cold water inlet and outlet circulation.
It is collected different receiver as the second/subsequent fraction.
(vii)Each fraction collected should be confirmed from known physical/chemical properties/characteristic. e.g.
Ethanol
Ethanol is a colourless liquid that has a characteristic smell .When it is put in a watch glass then ignited, it catches fire and burn with a blue flame.
Water
Water is a colourless liquid that has no smell/odour .When it is put in a watch glass then ignited, it does not catch fire.
Set up of apparatus
Industrial application of Fractional distillationOn a large scale,fractional distillation is used:
(i)Infractional distillation of crude oil in an oil refinery.
Crude oil is a mixture of many fractions. When heated in a furnace, the different fractions separate out according to their boiling point.
In Kenya,fractional distillation takes place at Changamwe in Mombasa.
(ii)In fractional distillation of air.
Air contain a mixture of three main useful gases which are condensed by coolin to very low temperature (-200oC) to form a liquid.
The liquid is then heated.Nitrogen is the most volatile(-196oC) and thus comes out as the first fraction. Argon (at -186oC) is the second fraction.
Oxygen ( at -183oC) is the last fraction. The three gases are very useful industrial gases.
(f)Separation of immiscibles (Using a separating funnel)Two or more liquids that form layers on mixing are immiscible.Immiscible mixture arrange themselves according to their densities
i.e The denser liquid sink to the bottom. The less dense liquid floats on the denser one. Immicible mixtures can be separated from each other by using a separating funnel.
Experiment: To separate an immiscible mixture of paraffin and water.
Procedure
Place about 100cm3 of water into a 250cm3 beaker. Add about 100cm3 of paraffin into the beaker. Stir.
Transfer the mixture into a separating funnel.
Allow to settle for about one minute. Open the tap, run out the lower layer out slowly into a clean beaker.
Close the tap when the upper layer is very close to the tap.
Run out the intermediate small amount of the mixture near the tap into a beaker.
Discard it. Run out the remaining upper layer into a fresh beaker.
Place a portion of upper and lower layer into a watch glass separately after separating each.Ignite.
Observation
Water and paraffin are both colourless liquids.
Two layers are formed on mixing.
Colourless odourless liquid collected first. It does not catch fire.
A colourless liquid with characteristic smell collected later/second. It catches fire and burn with a yellow smoky flame.
Explanation
Water and paraffin are immiscible. Water is denser than paraffin.When put in a separating funnel, paraffin float on water. On opening the tap, water runs out.
A mixture of water and paraffin at the junction of the two is discarded. It is not pure.
Set up of apparatus
(g)Sublimation/depositionSome solids on heating do not melt to a liquid but change directly to a gas. The process by which a solid changes to a gas is called sublimation.
The gas cools back and changes directly to a solid.The process by which a gas changes to a solid is called deposition.
Sublimation and deposition therefore are the same but opposite processes.
Some common substances that undergosublimation/ deposition include:(i)Iodine (ii)Carbon(IV)oxide (iii)Camphor (iv) ammonium
chloride (v)Iron(III)chloride (vi)Aluminium(III)chloride
(vii) benzoic acid If a mixture has any of the above as a component, then on heating it will change to a gas and be deposited away from the source of heating.
Procedure
Place about one spatula full of ammonium chloride crystals into a clean dry 100cm3 beaker.
Add equal amount of sodium chloride crystals into the beaker. Swirl to mix.
Place the beaker on a tripod stand.
Put about 100cm3 of water into another beaker. Place carefully the beaker containing water on top of the beaker containing the solid mixture. Light/ignite a burner and heat the solid.
Set up of apparatus:
Observation(i)With ammonium chloride/common salt mixture
White fumes produced .
White sublimate deposited
Colourless residue left
(ii)With Iodine/common salt mixture
Purple fumes produced .
Dark grey sublimate deposited
Colourless residue left
Explanation
(i)On heating a mixture of ammonium chloride and common salt, a white fumes of ammonium chloride is produced.
The white fumes solidify as white sublimate on the cooler parts.Common salt remains as residue.
Chemical equation:
Ammonium chloride solid Ammonium chloride gas
NH4Cl(s) NH4Cl(g)
(ii)On heating a mixture of Iodine and common salt, a purple fumes of Iodine vapour is produced. The purple fumes solidify as dark grey sublimate on the cooler parts.Common salt remains as residue.
Chemical equation:
Iodine solid Iodine gas
I2(s) I2 (g)
(h)Chromatography
Chromatography is a method of separating components of a solution mixture by passing it through a medium where the different components move at different rates.
The medium through which the solution mixture is passed is called absorbent material.
Paper chromatography is a method of separating coloured dyes by using paper as the absorbent material.
Since dyes are insoluble/do not dissolve in water,ethanol and propanone are used as suitable solvents for dissolving the dye.
Practically, a simple paper chromatography involve placing a dye/material on the absorbent material, adding slowly a suitable soluble solvent on the dye/material using a dropper, the solvent spread out on the absorbent material carrying the soluble dye away from the origin.
The spot on which the dye is initially/originally placed is called baseline. The farthest point the solvent spread is called solvent front.
The farthest a dye can be spread by the solvent depend on:
(i)density of the dye-the denser the dye, the less it spread from the basely ne by the solvent.
(ii) Stickiness of the dye-some dyes sticks on the absorbent material more than other thus do not spread far from baseline.
Experiment: To investigate the colours in ink
Procedure
Method 1
Place a filter paper on a an empty beaker. Put a drop of black/blue ink in the centre of the filter paper. Wait for about one minute for the ink drop to spread.
Using a clean teat pipette/dropper add one drop of ethanol/propanone.
Wait for about one minute for the ink drop to spread further.
Add about twenty other drops of ethanol waiting for about one minute before each addition. Allow the filter paper to dry.
Experiment: To investigate the colours in ink
Procedure
Method 2
Cut an8 centimeter thin strip of a filter paper.
At about 3cm on the strip,place a drop of ink.
Place the filter paper in a 10cm length boiling tube containing 5cm3 of ethanol.
Ensure the cut strip of the filter paper just dips into the ethanol towards the ink mark.
Cover the boiling tube.
Wait for about twenty minutes.
Remove the boiling tube and allow the filter paper to dry.
Set up of apparatus
Method 1
Set up of apparatusMethod 2
ExplanationWhen a drop of ink is placed on an absorbent material it sticks. On adding an eluting solvent, it dissolves the dye spread out with it.
The denser and sticky pure dye move least.
The least dense/sticky pure dye move farthest.
A pure dye will produce the same chromatogram/spot if the same eluting solvent is used on the same absorbent material. Comparing the distance moved by a pure dye with a mixture ,the coloured dyes in a mixture can be deduced as below:
Example 1
The chromatogram of pure dyes A,B ,C and a dye mixture D is shown below Determine the pure dyes present in D. On the diagram show:
(i)the solvent front
(ii)baseline
(iii)the most soluble pure dye
(i) Solvent extractionSolvent extraction is a method of separating oil from nuts/seeds. Most nuts contain oil.
First the nuts are crushed to reduce their size and increase the surface area.
A suitable volatile solvent is added.
The mixture is filtered. The filtrate solvent is then allowed to crystallize leaving the oil/fat.
If a filter paper is rubbed/smeared with the oil/fat, it becomes translucent. This is the test for the presence of oil/fat.
Experiment: To extract oil from Macadamia nut seeds
Procedure
Crush Macadamia nut seeds form the hard outer cover .
Place the inner soft seed into a mortar. Crush(add a little sand to assist in crushing).
Add a little propanone and continue crushing.
Continue crushing and addinga little propanone until there is more liquid mixture than the solid.
Decant/filter.
Put the filtrate into an evaporating dish.
Vapourize the solvent using solar energy /sunlight.
Smear/rub a portion of the residue left after evaporation on a clean dry filter paper.
Observation /Explanation
Propanone dissolve fat/oil in the macadamia nuts. Propanone is more volatile(lower boiling point)than oil/fat.
In sunlight/solar energy, propanone evaporate/vapourize leaving oil/fat(has a higher boiling point).
Any seed like corn,wheat ,rice,soya bean may be used instead of macadamia seed.When oil/fat is rubbed/smeared on an opaque paper,it becomes translucent.
(j) Crystallization
Crystallization is the process of using solubility of a solute/solid to obtain the solute/solid crystals from a saturated solution by cooling or heating the solution.
A crystal is the smallest regular shaped particle of a solute. Every solute has unique shape of its crystals.
Some solutions form crystals when heated. This is because less solute dissolve at higher temperature.Some other solutions form crystals when cooled.
This is because less solute dissolve at lower temperature.
Experiment;To crystallize copper(II)sulphate(VI)solution
Procedure:
Place about one spatula full of hydrated copper sulphate(VI) crystals into 200cm3 of distilled water in a beaker.Stir.
Continue adding a little more of the hydrated copper sulphate(VI) crystals and stirring until no more dissolve. Decant/filter.
Cover the filtrate with a filter paper.Pierce and make small holes on the filter paper cover.
Preserve the experiment for about seven days.
Observation/Explanation
Large blue crystals formed
When hydrated copper(II)sulphate crystals are placed in water, they dissolve to form copper(II)sulphate solution.After some days water slowly evaporate leaving large crystals of copper(II)sulphate.
If the mixture is heated to dryness,small crystals are formed.
Physical/Temporary and Chemical changes
A physical/temporary change is one which no new substance is formed and is reversible back to original.
A chemical/permanent change is one which a new substance is formed and is irreversible back to original.
The following experiments illustrates physical and chemical changes
(a)Heating ice
Place about 10g of pure ice in a beaker. Determine its temperature.Record it at time “0.0” in the table below.
Heat the ice on a strong Bunsen flame and determine its temperature after every 60seconds/1minute to complete the table below:
Plot a graph of time against Temperature(y-axes)Explain the shape of your graph
Melting/freezing/fusion/solidification and boiling /vaporization /evaporation are the two physical processes.
Melting /freezing point of pure substances is fixed /constant.
The boiling point of pure substance depend on external atmospheric pressure.
Melting/fusion is the physical change of a solid to liquid.
Freezing is the physical change of a liquid to solid.
Melting/freezing/fusion/solidification are therefore two opposite but same reversible physical processes i.e
A (s) A(l)
Boiling/vaporization/evaporation is the physical change of a liquid to gas.
Condensation/ liquidification is the physical change of gas to liquid.
Boiling/vaporization/evaporation and condensation/ liquidification are therefore two opposite but same reversible physical processes i.e
B (l) B(g)
Practically
(i) Melting/liquidification/fusion involves heating a solid to weaken the strong bonds holding the solid particles together.
Solids are made up of very strong bonds holding the particles very close to each other (Kinetic Theory of matter).
On heating these particles gain energy/heat from the surrounding heat source to form a liquid with weaker bonds holding the particles close together but with some degree of freedom.
(ii)Freezing/fusion/solidification involves cooling a liquid to reform /rejoin the very strong bonds to hold the particles very close to each other as solid and thus lose their degree of freedom (Kinetic Theory of matter).
Freezing /fusion / solidification is an exothermic (-∆H)process that require particles holding the liquid together to lose energy to the surrounding.
(iii)Boiling/vaporization/evaporation involves heating a liquid to completely break/free the bonds holding the liquid particles together.
Gaseous particles have high degree of freedom (Kinetic Theory of matter).
Boiling /vaporization / evaporation is an endothermic (+∆H) process that require/absorb energy from the surrounding.
(iv)Condensation/liquidification is reverse process of boiling /vaporization / evaporation.
It involves gaseous particles losing energy to the surrounding to form a liquid.
Introduction to Acids,bases and Indicators
1.In a school laboratory:
(i)An acidmay be defined as a substance that turn litmus red.
(ii)A base may be defined as a substance that turn litmus blue.
Litmus is a lichen found mainly in West Africa. It changes its colour depending on whether the solution it is in, is basic/alkaline or acidic.It is thus able to identify/show whether another substance is an acid, base or neutral.
(iii)An indicator is a substance that shows whether another substance is a base/alkaline,acid or neutral.
2.Common naturally occurring acids include:
3.Most commonly used acids found in a school laboratory are not naturally occurring. They are manufactured. They are called mineral acids.Common mineral acids include:
4.Mineral acids are manufactured to very high concentration. They are corrosive(causes painful wounds on contact with the skin) and attack/reacts with garments/clothes/metals.In a school laboratory, they are mainly used when added a lot of water.
This is called diluting.Diluting ensures the concentration of the acid is safely low.
5. Bases are opposite of acids. Most bases do not dissolve in water.
Bases which dissolve in water are called alkalis.
Common alkalis include:
6. Indicators are useful in identifying substances which look-alike. An acid-base indicator is a substance used to identify whether another substance is alkaline or acidic. Anacid-base indicator works by changing to different colours in neutral, acidic and alkaline solutions/dissolved in water.Experiment:To prepare simple acid-base indicator
Procedure
(a)Place some flowers petals in a mortar. Crush them using a pestle. Add a little sand to assist in crushing.
Add about 5cm3 of propanone/ethanol and carefully continue grinding.
Add more 5cm3 of propanone/ethanol and continue until there is enough extract in the mortar.
Filter the extract into a clean 100cm3beaker.
(b)Place 5cm3 of filtered wood ash, soap solution, ammonia solution, sodium hydroxide, hydrochloric acid, distilled water, sulphuric(VI)acid, sour milk, sodium chloride, toothpaste and calcium hydroxide into separate test tubes.
(c)Put about three drops of the extract in (a)to each test tube in (b). Record the observations made in each case.
Sample observations
The plant extract is able to differentiate between solutions by their nature. It is changing to a similar colour for similar solutions.(i)Since lemon juice is a known acid, then sulphuric(VI)and hydrochloric acids are similar in nature with lemon juice because the indicator show similar colours.
They are acidic in nature.
(ii)Since sodium hydroxide is a known base/alkali, then the green colour of indicator shows an alkaline/basic solution.
(iii)Since pure water is neutral,then the orange colour of indicator shows neutral solutions.
7. In a school laboratory, commercial indicators are used. A commercial indicator is cheap, readily available and easy to store. Common indicators include: Litmus, phenolphthalein, methyl orange, screened methyl orange, bromothymol blue.
Experiment:
Using commercial indicators to determine acidic,basic/alkaline and neutral solutions
Procedure
Place 5cm3 of the solutions in the table below. Add three drops of litmus solution to each solution.
Repeat with phenolphthalein indicator, methyl orange, screened methyl orange and bromothymol blue.
The universal indicatorThe universal indicator is a mixture of other indicator dyes.
The indicator uses the pH scale.The pH scale shows the strength of bases and acids.
The pH scale ranges from 1-14.These numbers are called pH values:
(i)pH values 1,2,3 shows a substance is strongly acid
(ii)pH values 4,5,6 shows a substance is a weakly acid
(iii)pH value 7 shows a substance is a neutral
(iv)pH values 8,9,10,11 shows a substance is a weak base/alkali.
(v)pH values 12,13,14 shows a substance is a strong base/alkali
The pH values are determined from a pH chart.
The pH chart is a multicoloured paper with each colour corresponding to a pH value.i.e
(i)red correspond to pH 1,2,3 showing strongly acidic solutions.
(ii)Orange/ yellow correspond to pH 4,5,6 showing weakly acidic solutions.
(iii)Green correspond to pH 7 showing neutral solutions.
(iv)Bluecorrespond to pH 8,9,10,11 showing weakly alkaline solutions.
(v)Purple/dark bluecorrespond to pH 12,13,14 showing strong alkalis.
The universal indicator is available as:
(i)universal indicator paper/pH paper
(ii)universal indicator solution.
When determining the pH of a unknown solution using
(i)pH paper then the pH paper is dipped into the unknown solution.
It changes/turn to a certain colour.
The new colour is marched/compared to its corresponding one on the pH chart to get the pH value.
(ii)universal indicator solution then about 3 drops of the universal indicator solution is added into about 5cm3 of the unknown solution in a test tube.It changes/turn to a certain colour.
The new colour is marched/compared to its corresponding one on the pH chart to get the pH value.
Experiment:To determine the pH value of some solutions
(a)Place 5cm3 of filtered wood ash, soap solution, ammonia solution, sodium hydroxide, hydrochloric acid, distilled water, sulphuric(VI)acid, sour milk, sodium chloride, toothpaste and calcium hydroxide into separate test tubes.
(b)Put about three drops of universal indicator solution or dip a portion of a piece of pH paper into each. Record the observations made in each case.
(c)Compare the colour in each solution with the colours on the pH chart provided.Determine the pH value of each solution.
Sample observations
Note1.All the mineral acids Hydrochloric, sulphuric(VI)and nitric(V)acids are strong acids
2.Two alkalis/soluble bases ,sodium hydroxide and potassium hydroxide are strong
bases/alkali. Ammonia solution is a weak base/alkali.All other bases are weakly alkaline.
3.Pure/deionized water is a neutral soulution.
4.Common salt/sodium chloride is a neutral salt.
5. When an acid and an alkali/base are mixed, the final product have pH 7 and is neutral.
Properties of acids
(a)Physical properties of acids
1.Acids have a characteristic sour taste
2.Most acids are colourless liquids
3.Mineral acids are odourless. Organic acids have characteristic smell
4.All acids have pH less than 7
5.All acids turn blue litmus paper red,methyl orange red and phenolphthalein colourless.
6.All acids dissolve in water to form an acidic solution.Most do not dissolve in organic
solvents like propanone,kerosene,tetrachloromethane,petrol.
(b)Chemical properties of acids.
1.Reaction with metals
All acids react with a reactive metals to form a salt and produce /evolve hydrogen gas.
Metal + Acid -> Salt + Hydrogen gas
Experiment :reaction of metals with mineral acids.
(a)Place 5cm3 of dilute hydrochloric acid in a small test tube. Add 1cm length of polished magnesium ribbon.
Stopper the test tube using a thump. Light a wooden splint. Place the burning splint on top of the stoppered test tube. Release the thump stopper. Record the observations made.
(b)Repeat the procedure in (a)above using Zinc granules, iron filings, copper turnings, aluminium foil in place of Magnesium ribbon
(c)Repeat the procedure in (a) then (b) using dilute sulphuric(VI) acid in place of dilute hydrochloric acid.
Sample observations
(i)effervescence/bubbles produced/fizzing in all cases except when using copper
(ii)colourless gas produced in all cases except when using copper
(iii)gas produced extinguishes a burning wooden splint with an explosion/pop sound.
Explanation
Some metals react with dilute acids,while others do not. Metals which react with acids produces bubbles of hydrogen gas.
Hydrogen gas is a colourless gas that extinguishes a burning splint with a pop sound.
This shows acids contain hydrogen gas.
This hydrogen is displaced/removed from the acids by some metals like Magnesium, Zinc, aluminium,iron and sodium.
Some other metals like copper, silver, gold, platinum and mercury are not reactive enough to displace/remove the hydrogen from dilute acids.
Chemical equations
1. Magnesium + Hydrochloric acid -> Magnesium chloride + Hydrogen
Mg(s) + 2HCl (aq) ->MgCl2 (aq) + H2(g)
2. Zinc + Hydrochloric acid ->Zinc chloride + Hydrogen
Zn(s) + 2HCl (aq) ->ZnCl2 (aq) + H2(g)
3. Iron + Hydrochloric acid -> Iron(II) chloride + Hydrogen
Fe(s) + 2HCl (aq) -> FeCl2 (aq) + H2(g)
4. Aluminium + Hydrochloric acid -> Aluminium chloride + Hydrogen 2Al(s) + 3HCl (aq) ->AlCl3 (aq) + 3H2(g)
5. Magnesium + Sulphuric(VI)acid -> Magnesium sulphate(VI) + Hydrogen
Mg(s) + H2SO4 (aq) ->MgSO4 (aq) + H2(g)
6. Zinc + Sulphuric(VI)acid -> Zinc sulphate(VI) + Hydrogen
Zn(s) + H2SO4 (aq) ->ZnSO4 (aq) + H2(g)
7. Iron + Sulphuric(VI)acid -> Iron(II) sulphate(VI) + Hydrogen
Fe(s) + H2SO4 (aq) ->FeSO4 (aq) + H2(g)
8. Aluminium + Sulphuric(VI)acid -> Aluminium sulphate(VI) + Hydrogen
2Al(s) + 3H2SO4 (aq) ->Al2(SO4)3 (aq) + 3H2(g)
2.Reaction of metal carbonates and hydrogen carbonates with mineral acids.
All acids react with carbonates and hydrogen carbonates to form a salt, water and produce /evolve carbon(IV)oxide gas.
Metal carbonate + Acid -> Salt + Water + Carbon(IV)oxide gas
Metal hydrogen carbonate + Acid -> Salt + Water + Carbon(IV)oxide gas
Experiment :reaction of metal carbonates and hydrogen carbonates with mineral acids.
(a)Place 5cm3 of dilute hydrochloric acid in a small test tube. Add half spatula full of sodium carbonate. Stopper the test tube using a cork with delivery tube directed into lime water. Record the observations made. Test the gas also with burning splint.
(b)Repeat the procedure in (a)above using Zinc carbonate, Calcium carbonate, copper carbonate, sodium hydrogen carbonate, Potassium hydrogen carbonate in place of Sodium carbonate.
(c)Repeat the procedure in (a) then (b) using dilute sulphuric(VI) acid in place of dilute hydrochloric acid.
Set up of apparatus
Sample observations(i)effervescence/bubbles produced/fizzing in all cases.
(ii)colourless gas produced in all cases.
(iii)gas produced forms a white precipitate with lime water.
Explanation
All metal carbonate/hydrogen carbonate reacts with dilute acids to produce bubbles of carbon (IV)oxide gas.Carbon(IV)oxide gas is a colourless gas that extinguishes a burning splint.
When carbon (IV) oxide gas is bubbled in lime water, a white precipitate is formed.
Chemical equations
1. Sodium carbonate +Hydrochloric acid -> Sodium chloride + Carbon(IV)Oxide+ Water
Na2CO3(s) + 2HCl (aq) -> 2NaCl (aq) + H2O(g) + CO2 (g)
2. Calcium carbonate +Hydrochloric acid -> Calcium chloride + Carbon(IV)Oxide+ Water CaCO3(s) + 2HCl (aq) -> CaCl2 (aq) + H2O(g) + CO2 (g)
3. Magnesium carbonate +Hydrochloric acid -> Magnesium chloride + Carbon(IV)Oxide+ Water MgCO3(s) + 2HCl (aq) -> MgCl2 (aq) + H2O(g) + CO2 (g)
4. Copper carbonate +Hydrochloric acid ->Copper(II) chloride + Carbon(IV)Oxide+ Water CuCO3(s) + 2HCl (aq) -> CuCl2 (aq) + H2O(g) + CO2 (g)
5. Copper carbonate +Sulphuric(VI) acid ->Copper(II)sulphate(VI) + Carbon(IV)Oxide+ Water CuCO3(s) + H2SO4 (aq) -> CuSO4 (aq) + H2O(g) + CO2 (g)
6. Zinc carbonate +Sulphuric(VI) acid ->Zinc sulphate(VI) + Carbon(IV)Oxide+ Water
ZnCO3(s) + H2SO4 (aq) -> ZnSO4 (aq) + H2O(g) + CO2 (g)
7. Sodium hydrogen carbonate +Sulphuric(VI) acid ->Sodium sulphate(VI) + Carbon(IV)Oxide+ Water
NaHCO3(s) + H2SO4 (aq) -> Na2SO4 (aq) + H2O(g) + CO2 (g)
8. Potassium hydrogen carbonate +Sulphuric(VI) acid -> Potassium sulphate(VI) + Carbon(IV)Oxide+ Water
KHCO3(s) + H2SO4 (aq) -> K3SO4 (aq) + H2O(g) + CO2 (g) 9. Potassium hydrogen carbonate +Hydrochloric acid -> Potassium chloride + Carbon(IV)Oxide+ Water KHCO3(s) + HCl (aq) -> KCl (aq) + H2O(g) + CO2 (g)
10. Sodium hydrogen carbonate +Hydrochloric acid -> Sodium chloride + Carbon(IV)Oxide+ Water NaHCO3(s) + HCl (aq) -> NaCl (aq) + H2O(g) + CO2 (g)
3.Neutralization bybases/alkalis
All acids react with bases to form a salt and water only.
The reaction of an acid withmetal oxides/hydroxides(bases) to salt and water only is called neutralization reaction.
Since no effervescence/bubbling/fizzing take place during neutralization:
(i) the reaction with alkalis require a suitable indicator.
The colour of the indicator changes when all the acid has reacted with the soluble solution of the alkali (metal oxides/ hydroxides).
(ii) excess of the base is added to ensure all the acid reacts. The excess acid is then filtered off.
Experiment 1:reaction of alkali with mineral acids.
(i)Place about 5cm3 of dilute hydrochloric acid in a boiling tube.Add one drop of phenolphthalein indicator. Using a dropper/teat pipette, add dilute sodium hydroxide dropwise until there is a colour change.
(ii)Repeat the procedure with dilute sulphuric (VI)acid instead of hydrochloric acid.
(iii)Repeat the procedure with potassium hydroxide instead of sodium hydroxide.
Sample observation:
Colour of phenolphthalein change from colourless to pink in all cases.
Explanation
Bases/alkalis neutralize acids. Acids and bases/alkalis are colourless.
A suitable indicator like phenolphthalein change colour topink,when all the acid has been neutralized by the bases/alkalis.
Phenolphthalein change colour frompink,to colourless when all the bases/alkalis has been neutralized by the acid.
Chemical equation
Sodium oxide + Hydrochloric acid ->Sodium chloride + Water
Na2O(s) + HCl ->NaCl(aq) + H2O(l)
Potassium oxide + Hydrochloric acid ->Potassium chloride + Water
K2O(s) + HCl -> KCl(aq) + H2O(l)
Sodium hydroxide + Hydrochloric acid ->Sodium chloride + Water
NaOH(s) + HCl -> NaCl(aq) + H2O(l)
Ammonia solution+ Hydrochloric acid ->Ammonium chloride + Water
NH4OH(s) + HCl -> NH4Cl(aq) + H2O(l)
Potassium hydroxide + Hydrochloric acid -> Potassium chloride + Water
KOH(s) + HCl -> KCl(aq)
+ H2O(l) Sodium hydroxide + sulphuric(VI)acid ->Sodium sulphate(VI)+ Water
2NaOH(s) + H2SO4 -> Na2SO2 (aq)
+ 2H2O(l) Potassium hydroxide + sulphuric(VI)acid-> Potassium sulphate(VI)+ Water 2KOH(s) + H2SO4-> K2SO4 (aq) + 2H2O(l)
Ammonia solution+ sulphuric(VI)acid ->Ammonium sulphate(VI)+ Water 2NH4OH(s) + H2SO4 -> ( NH4)2SO4 (aq)
+ 2H2O(l) Magnesium hydroxide + sulphuric(VI)acid ->Magnesium sulphate(VI) + Water Mg(OH)2(s) + H2SO4 -> MgSO4 (aq) + 2H2O(l)
Magnesium hydroxide + Hydrochoric acid ->Magnesium chloride + Water Mg(OH)2(s) + HCl(aq) -> MgCl2 (aq) + 2H2O(l)
The Atmosphere.
1.The atmosphere is made up of air. Air is a mixture of colourless , odourless gases which is felt as wind(air in motion).
All living things breath in air for respiration . Plants use air for respiration and photosynthesis.
2.The main gases present in the atmosphere/air:
3. The following experiments below shows the presence and composition of the gases in air/atmosphere(a)To find the composition of air supporting combustion using a candle stick
Procedure
Measure the length of and empty gas jar M1.
Place a candle stick on a petri dish.
Float it on water in basin/trough.
Cover it with the gas jar. Mark the level of the water in the gas jar M2.
Remove the gas jar.
Light the candle sick. Carefully cover it with the gas jar.
Observe for two minutes. Mark the new level of the water M3.
Set up of apparatus
Sample observationsCandle continues to burn then extinguished/goes off
Level of water in the gas jar rises after igniting the candle
Length of empty gas jar = M1= 14cm
Length of gas jar without water before igniting candle = M2= 10 cm
Length of gas jar with water before igniting candle = M1 -M2=14- 10 = 4cm
Length of gas jar with water after igniting candle = M3= 8cm
Length of gas jar without water after igniting candle = M1 – M3= 10 -8 = 2 cm
Explanation
Candle burns in air. In a closed system(vessel),the candle continues to burn using the part of air that support burning/combustion. This is called the active part of air.
The candle goes off/extinguished when all the active part of air is used up.
The level of the water rises to occupy the space /volume occupied by the used active part of air.
The experiment is better when very dilute sodium/potassium hydroxide is used instead of water .
Dilute Potassium/ sodium hydroxide absorb Carbon(IV)oxide gas that come out from burning/combustion of candle stick.
From the experiment above the % composition of the:
(i)active part of air can be calculated:
M2– M3x 100% => 10- 8x 100% = 20%
M2 10cm
(ii)inactive part of air can be calculated:
100% -20% = 80% // M3=> 8x 100%= 80%
M210cm
(b)To find the composition of active part of air using heated copper turnings.
Procedure
Clamp a completely packed/filled open ended glass tube with copper turnings.Seal the ends with glass/cotton wool. Label two graduated syringes as “A” and “B” Push out air from syringe “A”. Pull in air into syringe “B”.
Attach both syringe “A” and “B” on opposite ends of the glass tube.
Determine and record the volume of air in syringe “B” V1.
Heat the glass tube strongly for about three minutes.
Push all the air slowly from syringe “B” to syringe “A” as heating continues.
Push all the air slowly from syringe “A” back to syringe “B” and repeatedly back and forth.
After about ten minutes, determine the new volume of air in syringe “B” V1
Set up of apparatus
Sample observationsColour change from brown to black
Volume of air in syringe “B” before heating V1 = 158.0 cm3
Volume of air in syringe “B” after heating V2 = 127.2 cm3
Volume of air in syringe “B” used by copper V1 – V2= 30.8cm3
Sample questions
1.What is the purpose of
(i) glass/cotton wool
To prevent/stop copper turnings from being blown into the syringe/out of the glass tube
(ii) passing air through the glass tube repeatedly
To ensure all the active part of air is used up
(iii) passing air through the glass tube slowly
To allow enough time of contact between the active part of and the heated copper turnings.
2. State and explain the observations made in the glass tube.
Colour change from brown to black Brown copper metal reacts with the active part of air/oxygen to form black copper(II)oxide.
Chemical equation
Copper + Oxygen -> Copper(II)oxide
2Cu(s) + O2(g) -> 2CuO(s)
The reaction reduces the amount/volume of oxygen in syringe “B” leaving the inactive part of air. Copper only react with oxygen when heated.
3. Calculate the % of
(i)active part of air
% active part of air = V1 – V2x 100% => 30.8cm3 x 100% =19.493%
V1 158.0cm3
(ii) inactive part of air
Method 1
% inactive part of air = V2 x 100% =>127.2cm3 x 100% = 80.506%
V1 158.0cm3
Method 2
% inactive part of air = 100% -% active part of air
=> 100 % – 19.493 % = 80.507%
4.The % ofactive part of air is theoretically higher than the above while % of inactive part of air is theoretically lower than the above. Explain.
Not all the active part of air reacted with copper
5.State the main gases that constitute:
(a)active part of air.
Oxygen
(b)inactive part of air
Nitrogen, carbon(IV)oxide and noble gases
6.If the copper turnings are replaced with magnesium shavings the % of active part of air obtained is extraordinary very high. Explain.
Magnesium is more reactive than copper. The reaction is highly exothermic.
It generates enough heat for magnesium to react with both oxygen and nitrogen in the air.
A white solid/ash mixture of Magnesium oxide and Magnesium nitride is formed.
This considerably reduces the volume of air left after the experiment.
Chemical equation Magnesium + Oxygen -> magnesium (II)oxide
2Mg(s) + O2(g) -> 2MgO(s)
Magnesium + Nitrogen -> magnesium (II)nitride
3Mg(s) + N2(g) -> Mg3N2 (s)
(c)To find the composition of active part of air using alkaline pyrogallol.
Procedure
Measure about 2cm3of dilute sodium hydroxide into a graduated gas jar. Record the volume of the graduated cylinder V1.
Place about two spatula end full of pyrogallol/1,2,3-trihydroxobenzene into the gas jar.2Immediately place a cover slip firmly on the mouth of the gas jar. Swirl thoroughly for about two minutes.
Invert the gas jar in a trough/basin containing water.Measure the volume of air in the gas jar V2 Sample observations
Colour ofpyrogallol/1,2,3-trihydroxobenzene change to brown.
Level of water in gas jar rises when inverted in basin/trough.
Volume of gas jar/air in gas jarV1= 800cm3 Volume of gas jar/air in gas jarafter shaking with alkaline pyrogallol/1,2,3-trihydroxobenzeneV2= 640 cm3
Sample questions
1.Which gas is absorbed by alkaline pyrogallol/1,2,3-trihydroxobenzene
Oxygen
2.Calculate the
(i) % of active part of air
V1-V2 x 100% =>(800cm3 – 640 cm3) x 100% = 20%
V1 800cm3
(ii) % of inactive part of air V2 x 100% => 640 cm3 x 100% = 80%
V1 800cm3
(d)To establish the presence of carbon(IV)oxide in air using lime water Pass tap water slowly into an empty flask as in the set up below
Sample observation questions1.What is the purpose of paper cover?
To ensure no air enters into the lime water.
2.What happens when water enters the flask?
It forces the air from the flask into the lime water.
3.What is observed when the air is bubbled in the lime water
A white precipitate is formed. The white precipitate dissolves on prolonged bubbling of air.
4.(a) Identify the compound that form:
(i)lime water
Calcium hydroxide / Ca(OH)2
(ii)white precipitate
Calcium carbonate/ CaCO3
(iii)when the white precipitate dissolves
Calcium hydrogen carbonate/ CaHCO3
(b)Write the chemical equation for the reaction that tale place when: (i)white precipitate is formed
Calcium hydroxide + carbon(IV)oxide ->Calcium carbonate + water
Ca(OH)2(aq) +CO2(g) ->CaCO3(s) +H2O(l)
(ii)white precipitate dissolves
Calcium carbonate+ water+ carbon(IV)oxide ->Calcium hydrogen carbonate
CaCO3(s) +H2O(l)+CO2(g)->CaHCO3(aq)
5. State the chemical test for the presence of carbon(IV)oxide gas based on 4(a) and (b)above:
Carbon(IV)oxide forms a white precipitate with lime water that dissolves in excess of the gas.
6. State the composition of carbon(IV)oxide gas by volume in the air.
About 0.03% by volume
Oxygen.
a) Occurrence.
1.Fifty 50% of the earths crust consist of Oxygen combined with other elements e.g.oxides of metals
2.About 70% of the earth is water made up of Hydrogen and Oxygen.
3.About 20% by volume of the atmospheric gases is Oxygen that form the active part of air.
b)School laboratory preparation.
Oxygen was first prepared in 1772 by Karl Scheele and later in 1774 by Joseph Priestly.
It was Antony Lavoisier who gave it the name “Oxygen”
Procedure
Method 1: Using Hydrogen peroxide
Half fill a trough/basin with tap water.
Place a bee hive shelf/stand into the water.
Completely fill the a gas jar with water and invert in onto the bee hive shelf/stand.
Clamp a round bottomed flask and set up the apparatus as below.
Collect several gas jars of Oxygen covering each sample.Sample observation questions
1.What is observed when the hydrogen peroxide is added into the flask
Rapid effervescence/bubbling/fizzing
2.Describe the colour and smell of the gas
Colourless and odourless.
3.(a)Name the method of gas collection used.
-Over water
-Upward delivery
-Down ward displacement of water
(b)What property of Oxygen make it to be collected using the method above
-Slightly soluble in water
4.What is the purpose of manganese(IV)oxide?
Manganese(IV)oxide is catalyst.
A catalyst is a substance that speeds up the rate of a chemical reaction but remain chemically unchanged at the end of the reaction.
Hydrogen peroxide decomposes slowly to form water and Oxygen gas.
A little Manganese(IV)oxide speeds up the rate of decomposition by reducing the time taken for a given volume of Oxygen to be produced.
5.Write the equation for the reaction.
Hydrogen peroxide -> Water+ Oxygen 2H2O2 (aq) -> 2H2 O (l) + O2 (g)
6.Lower a glowing splint slowly into a gas jar containing Oxygen gas.State what is observed.
The glowing splint relights/rekindles
Oxygen relights/rekindles a glowing splint.
This is the confirmatory test for the presence of Oxygen gas
Method 1: Using Sodium peroxide
Half fill a trough/basin with tap water. Add four drops of phenolphthalein indicator. Place abee hive shelf/stand into the water.
Completely fill a gas jar with water and invert in onto the bee hive shelf/stand. Clamp a round bottomed flask and set up the apparatus as below.
Collect several gas jars of Oxygen covering each sample.Sample observation questions
1.What is observed when water is added
(i)into the flask containing sodium peroxide
Rapid effervescence/bubbling/fizzing
(ii)phenolphththalein
Remains colourless/Phenolphthalein indicator is colourless in neutral solution
2.Describe the colour and smell of the gas
Colourless and odourless.
3.(a)Name the method of gas collection used.
-Over water.Oxygen is slightly soluble in water.
4. Test the gas by lowering a glowing splint slowly into a gas jar containing the prepared sample.
The glowing splint relights/rekindles. This confirms the presence of Oxygen gas 5.Write the equation for the reaction.
Sodium peroxide + Water -> Sodium hydroxide +Oxygen
2Na2O2 (aq) + 2H2O (l) -> 4NaOH(aq) + O2 (g)
1. Test the gas by lowering a glowing splint slowly into a gas jar containing the prepared sample.The glowing splint relights/rekindles.
This confirms the presence of Oxygen gas
2.Write the equation for the reaction. Potassium Chlorate(V) -> Potassium Chloride + Oxygen
2KClO3 (aq) -> 2KCl(aq) + 3O2 (g)
3.What is the purpose of manganese(IV)oxide?
Manganese(IV)oxide is catalyst.
A catalyst is a substance that speeds up the rate of a chemical reaction but remain chemically unchanged at the end of the reaction.
Potassium Chlorate(V) decomposes slowly to form potassium chloride and Oxygen gas.
A little Manganese(IV)oxide speeds up the rate of decomposition by reducing the time taken for a given volume of Oxygen to be produced.
(c)Uses of Oxygen
1. Oxygen is put in cylinders for use where natural supply is not sufficiently enough. This is mainly in:
(i)Mountain climbing/Mountaineering-at high altitudes, the concentration of air/oxygen is low.
Mountain climbers must therefore carry their own supply of oxygen for breathing.
(ii)Deep sea diving-Deep sea divers carry their own supply of Oxygen.
(iii)Saving life in hospitals for patients with breathing problems and during anaethesia.
2.A mixture of oxygen and some other gases produces a flame that is very hot.
(i) Oxy-acetyline/ethyne flame is produced when Ethyne/acetylene gas is burnt in pure oxygen.
The flame has a temperature of about 3000oC.It is used for welding/cuttingmetals.
(ii)Oxy-hydrogen flame is produced when Hydrogen is burn in pure oxygen. The flame has a temperature of about 2000oC.It is used also for welding/cuttingmetals.
3.Oxy-hydrogen mixture is used as rocket fuel
4. A mixture of charcoal , petrol and liquid Oxygen is an explosive.
(d)Chemical properties of Oxygen /combustion.
Oxygen is a very reactive non metal.Many elements react with oxygen through burning to form a group of compounds called Oxides.
Burning/combustion is the reaction of Oxygen with an element/substances.
Reaction in which a substance is added oxygen is called Oxidation reaction.
Burning/combustion is an example of an oxidation reaction.
Most non metals burns in Oxygen/air to form an Oxide which in solution / dissolved in water is acidic in nature.
They turn blue litmus red.e.g. Carbon(IV)oxide/CO2 ,Nitrogen(IV)oxide/NO2 ,Sulphur(IV)oxide/SO2
Some non metals burns in Oxygen/air to form an Oxide which in solution / dissolved in water is neutral in nature. They don’t turn blue or red litmus.e.g.
Carbon(II)oxide/CO, Water/H2 O.
All metals burns in Oxygen/air to form an Oxide which in solution/dissolved in water is basic/alkaline in nature. They turn red litmus blue.e.g.
Magnesium oxide/MgO, Sodium Oxide/ Na2 O ,Copper(II)oxide/CuO Elements/substances burn faster in pure Oxygen than in air.
Air contains the inactive part of air that slows the rate of burning of substances/elements.
(i)Reaction of metals with Oxygen/air
The following experiments show the reaction of metals with Oxygen and air.
I. Burning Magnesium
Procedure
(a)Cut a 2cm length piece of magnesium ribbon. Using a pair of tongs introduce it to a Bunsen flame. Remove it when it catches fire. Observe.
Place the products in a beaker containing about 5cm3 of water.
Test the solution/mixture using litmus papers
(b)Cut another 2cm length piece of magnesium ribbon. Using a pair of tongs introduce it to a Bunsen flame.When it catches fire, lower it slowly into a gas jar containing Oxygen. Place about 5cm3 of water into the gas jar.
Test the solution/mixture using litmus papers.Test the solution/mixture using litmus papers
Observations
(a)In air
Magnesium burns with a bright blindening flame in air forming white solid/ash /powder. Effervescence/bubbles/ fizzing Pungent smell of urine.
Blue litmus paper remains blue. Red litmus paper turns blue
(b) In pure Oxygen
Magnesium burns faster with a very bright blindening flame pure oxygenforming white solid/ash /powder.
No effervescence/bubbles/ fizzing.
No pungent smell of urine. Blue litmus paper remains blue. Red litmus paper turns blue
Explanation
Magnesium burns in air producing enough heat energy to react with both Oxygen and Nitrogen to form Magnesium Oxide and Magnesium nitride.
Both Magnesium Oxide and Magnesium nitride are white solid/ash /powder.
Chemical equations
Magnesium + Oxygen ->Magnesium Oxide
2Mg(s) + O2(g) -> 2MgO(s)
Magnesium + Nitrogen ->Magnesium Nitride
3Mg(s) + N2(g) ->Mg3N2 (s) Magnesium Oxide dissolves in water to form a basic/alkaline solution of Magnesium hydroxide Chemical equations
MagnesiumOxide + Water ->Magnesium hydroxide
2Mg(s) + O2(l) -> 2MgO(s)
Magnesium Nitride dissolves in water to form a basic/alkaline solution of Magnesium hydroxide and producing Ammonia gas.Ammonia is also an alkaline/basic gas that has a pungent smell of urine.
Chemical equations
Magnesium Nitride + Water ->Magnesium hydroxide + Ammonia gas
Mg3N2 (s)+ 6H2O (l) -> 3Mg(OH)2(aq) + 2NH3(g)
II. Burning Sodium
Procedure
(a)Carefully cut a very small piece of sodium . Using a deflagrating spoon introduce it to a Bunsen flame. Remove it when it catches fire. Observe.
Place the products in a beaker containing about 20cm3 of water. Test the solution/mixture using litmus papers
(b) Carefully cut another very small piece of sodium. Using a deflagrating spoon introduce it to a Bunsen flame.When it catches fire, lower it slowly into a gas jar containing Oxygen. Place about 20 cm3 of water into the gas jar.Test the solution/mixture using litmus papers.Test the solution/mixture using litmus papers
Observations
(a)In air
Sodium burns with a yellow flame in air forming a black solid. Blue litmus paper remains blue. Red litmus paper turns blue
(b) In pure Oxygen
Sodium burns faster with a golden yellow flame in pure oxygen forming a yellow solid.Effervescence/bubbles/ fizzing.
Gas produced relights glowing splint.
Blue litmus paper remains blue. Red litmus paper turns blue.
Explanation
(a)Sodium burnsin air forming black Sodium Oxide
Chemical equations
Sodium + Oxygen/air -> Sodium Oxide
4Na(s) + O2(g) -> 2Na2O(s)
Sodium Oxide dissolves in water to form a basic/alkaline solution of Sodium hydroxide
Chemical equations
Sodium Oxide + Water ->Sodium hydroxide
Na2O(s) + H2O (l) -> 2NaOH(aq)
(b)Sodium burns in pure oxygen forming yellow Sodium peroxide
Chemical equations
Sodium + Oxygen -> Sodium peroxide 2Na(s) + O2(g) -> Na2O2 (s) Sodium peroxide dissolves in water to form a basic/alkaline solution of Sodium hydroxide. Oxygen is produced.
Chemical equations
Sodium Oxide + Water -> Sodiumhydroxide + Oxygen 2Na2O2 (s) + 2H2O (l) -> 4NaOH(aq) + O2 (l)
III. Burning Calcium
Procedure
(a)Using a pair of tongs hold the piece of calcium on a Bunsen flame. Observe.
Place the products in a beaker containing about 2cm3 of water. Test the solution/mixture using litmus papers
(b)Using a pair of tongs hold another piece of calcium on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas .Observe.
Place about 2cm3 of water.Swirl.
Test the solution/mixture using litmus papers
Observations
(a)In air
Calcium burns with difficulty producing a faint red flame in air forming a white solid. Blue litmus paper remains blue. Red litmus paper turns blue
(b) In pure Oxygen
Calcium burns with difficulty producing a less faint red flame Oxygen forming a white solid.
Blue litmus paper remains blue. Red litmus paper turns blue
Explanation
(a)Calcium burns in air forming white calcium Oxide. Calcium Oxide coat/cover the calcium preventing further burning.
Chemical equations
Calcium + Oxygen/air -> calcium Oxide
2Ca(s) + O2(g) -> 2CaO(s)
Small amount of Calcium Oxide dissolves in water to form a basic/alkaline solution of Calcium hydroxide.The common name of Calcium hydroxide is lime water.
Chemical equations
Calcium Oxide + Water ->Calcium hydroxide
CaO(s) + H2O (l) -> Ca(OH)2 (aq)
IV. Burning Iron Procedure
(a)Using a pair of tongs hold the piece of Iron wool/steel wire on a Bunsen flame.
Observe.
Place the products in a beaker containing about 2cm3 of water. Test the solution/mixture using litmus papers (b)Using a pair of tongs hold another piece of Iron wool/steel wire on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas .
Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers
Observations (a)In air
Iron wool/steel wire burns producing a Orange flame in air forming a brown solid.
Blue litmus paper remains blue. Red litmus paper turns faint blue
(b) In pure Oxygen
Iron wool/steel wire burns producing a golden Orange flame in Oxygen forming a Brown solid. Blue litmus paper remains blue. Red litmus paper turns faint blue
Explanation
(a)Iron burns in air forming brown Iron(III) Oxide
Chemical equations
Iron + Oxygen/air -> Iron(III)Oxide 4Fe(s) + 3O2(g) -> 2Fe2O3(s)
Very small amount of Iron(III)Oxide dissolves in water to form a weakly basic/alkaline brown solution of Iron(III) hydroxide.
Chemical equations
Calcium Oxide + Water ->Iron(III)hydroxide Fe2O3(s) + 3H2O (l) ->2Fe(OH)3 (s)
V. Burning Copper
Procedure
(a)Using a pair of tongs hold the piece of copper turnings/shavings on a Bunsen flame. Observe.
Place the products in a beaker containing about 2cm3 of water. Test the solution/mixture using litmus papers
(b)Using a pair of tongs hold another piece of Copper turnings/shavings on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas .
Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers
Observations
(a)In air
Copper turnings/shavings burns with difficulty producing a green flame in air forming a black solid.
Blue litmus paper remains blue. Red litmus paper turns faint blue
(b) In pure Oxygen
Copper turnings/shavings burns less difficulty producing a green flame in Oxygen forming a Brown solid.
Blue litmus paper remains blue. Red litmus paper turns faint blue
Explanation
(a)Copper burns in air forming black Copper(II) Oxide
Chemical equations
Copper + Oxygen/air -> Copper(II)Oxide
2 Cu(s) + O2(g) -> 2CuO(s)
Very small amount of Copper(II)Oxide dissolves in water to form a weakly basic/alkaline blue solution of Copper(II) hydroxide.
Chemical equations
Copper(II) Oxide + Water -> Copper(II)hydroxide
CuO(s) + H2O (l) ->Cu(OH) 2 (s)
(i)Reaction of non metals with Oxygen/air
The following experiments show the reaction of non metals with Oxygen and air.
I. Burning Carbon
Procedure
(a)Using a pair of tongs hold a dry piece of charcoal on a Bunsen flame. Observe. Place the products in a beaker containing about 2cm3of water.
Test the solution/mixture using litmus papers
(b)Using a pair of tongs hold another piece of dry charcoal on a Bunsen flame. Quickly lower it into a gas jar containing Oxygen gas .
Observe.
Place about 2cm3 of water. Swirl. Test the solution/mixture using litmus papers Observations
-Carbon chars then burns with a blue flame
-Colourless and odourless gas produced
-Solution formed turn blue litmus paper faint red.
Red litmus paper remains red.
Explanation
Carbon burns in air and faster in Oxygen with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
Carbon burns in limited supply of air with a blue non-sooty/non-smoky flame forming Carbon (IV) oxide gas.
Carbon (IV) oxide gas dissolve in water to form weak acidic solution of Carbonic (IV)acid.
Chemical Equation
Carbon + Oxygen -> Carbon(IV)oxide
(excess air/oxygen)
C(s) + O2(g) -> CO2(g) (in excess air)
Carbon + Oxygen -> Carbon(II)oxide
(limited air/oxygen)
2C(s) + O2(g) -> 2CO(g) (in limited air)
Carbon(IV)oxide + Water -> Carbonic(IV)acid CO2(g) + H2O (l) -> H2CO3 (aq) (very weak acid)
II. Burning Sulphur
Procedure
(a)Using a deflagrating spoon place sulphur powder on a Bunsen flame.
Observe.
Place the products in a beaker containing about 3cm2 of water. Test the solution/mixture using litmus papers
(b)Using a deflagrating spoon place sulphur powder on a Bunsen flame. Slowly lower it into a gas jar containing Oxygen gas.Observe.
Place about 5cm3 of water. Swirl. Test the solution/mixture using litmus papers.
Observations
-Sulphur burns with a blue flame
-Gas produced that has pungent choking smell
-Solution formed turn blue litmus paper faint red.
Red litmus paper remains red.
Explanation
Sulphur burns in air and faster in Oxygen with a blue non-sooty/non-smoky flame forming Sulphur (IV) oxide gas.
Sulphur (IV) oxide gas dissolve in water to form weak acidic solution of Sulphuric (IV)acid.
Chemical Equation
Sulphur+ Oxygen ->Sulphur(IV)oxide
S(s) + O2(g) -> SO2(g) (in excess air) Sulphur(IV)oxide+ Water ->Sulphuric(IV)acid SO2(g) + H2O (l) -> H2SO3 (aq) (very weak acid)
III. Burning Phosphorus
Procedure
(a)Remove a small piece of phosphorus from water and using a deflagrating spoon (with a lid cover)place it on a Bunsen flame.
Observe.
Carefully put the burning phosphorus to cover gas jar containing about 3cm3 of water.
Test the solution/mixture using litmus papers
(b)Remove another small piece of phosphorus from water and using a deflagrating spoon (with a lid cover)place it on a Bunsen flame.
Slowly lower it into a gas jar containing Oxygen gas with about 5 cmsub>3 of water.
Observe.
Swirl. Test the solution/mixture using litmus papers.
Observations
-Phosphorus catches fire before heating on Bunsen flame
-Dense white fumes of a gas produced that has pungent choking poisonous smell -Solution formed turn blue litmus paper faint red.
Red litmus paper remains red.
Explanation
Phosphorus is stored in water.On exposure to air it instantaneously fumes then catch fire to burn in air and faster in Oxygen with a yellow flame producing dense white acidic fumes of Phosphorus(V) oxide gas.
Phosphoric(V) oxide gas dissolve in water to form weak acidic solution of Phosphoric (V)acid.
Chemical Equation
Phosphorus+ Oxygen ->Phosphorous(V)oxide
4P(s) + 5O2(g) -> 2P2(O5((s) Phosphorous(V)oxide + Water ->Phosphoric(V)acid P2O5((s)+3H2(O (l) -> 2H3(PO4( (aq) (very weak acid)
(e) Reactivity series/competition for combined Oxygen.
The reactivity series is a list of elements/metals according to their affinity for oxygen. Some metals have higher affinity for Oxygen than others.
A metal/element with higher affinity for oxygen is placed higher/on top of the one less affinity.
The complete reactivity series of metals/elements
Metals compete for combined Oxygen. A metal/element with higher affinity for oxygen removes Oxygen from a metal lower in the reactivity series/less affinity for Oxygen.When a metal/element gains/acquire Oxygen, the process is called Oxidation.
When a metal/element donate/lose Oxygen, the process is called Reduction.
An element/metal/compound that undergo Oxidation is called Reducing agent.
An element/metal/compound that undergo Reduction is called Oxidizing agent.
A reaction in which both Oxidation and Reduction take place is called a Redox reaction. Redox reaction between Magnesium and copper(II)Oxide
Procedure
Place about 2g of copper(II)oxide in a crucible with a lid. Place another 2g of Magnesium powder into the crucible. Mix thoroughly.
Cover the crucible with lid. Heat strongly for five minutes.
Allow the mixture to cool. Open the lid. Observe.
Observation
Colour change from black to brown. White solid power formed.
Explanation
Magnesium is higher in the reactivity series than Copper. It has therefore higher affinity for Oxygen than copper.
When a mixture of copper(II)oxide and Magnesium is heated, Magnesium reduces copper(II)oxide to brown copper metal and itself oxidized to Magnesium oxide.
Magnesium is the reducing agent because it undergoes oxidation process.
Copper(II)oxide is the oxidizing agent because it undergo redox reduction process.
The mixture should be cooled before opening the lid to prevent hot brown copper from being reoxidized back to black copper(II)oxide.
The reaction of Magnesium and Copper(II)oxide is a reaction
Chemical equation
1. Copper (II)oxide + Magnesium -> Magnesium oxide + Copper
(black) (white ash/solid) (brown)
CuO(s) + Mg(s) -> MgO(s) + Cu(s)
(Oxidizing Agent) (Reducing Agent)
2. Zinc (II)oxide + Magnesium -> Magnesium oxide +Zinc
(yellow when hot) (white ash/solid) (grey)
ZnO(s) + Mg(s) -> MgO(s) + Zn(s)
(Oxidizing agent) (Reducing agent)
3. Zinc (II)oxide + Carbon -> Carbon(IV) oxide gas + Zinc
(yellow when hot) (colourless gas) (grey)
ZnO(s) + C(s) -> CO2(g) + Zn(s)
(Oxidizing agent) (Reducing agent)
The reactivity series is used during extraction of metals from their ore.An ore is a rock containing mineral element which can be extracted for commercial purposes. Most metallic ores occur naturally as:
(i)oxides combined with Oxygen
(ii)sulphides combined with Sulphur
(iii)carbonates combined with carbon and Oxygen.
Metallic ores that naturally occur as metallic sulphides are first roasted in air to form the corresponding oxide. Sulphur(IV)oxide gas is produced. e.g.
Copper(I) sulphide + Oxygen -> Copper(I)Oxide + Sulphur(IV)oxide
Cu2S(s) + O2(g) -> 2Cu(s) + SO2(g)
Zinc(II) sulphide + Oxygen -> Zinc(II)Oxide + Sulphur(IV)oxide
ZnS(s) + O2(g) -> Zn(s) + SO2(g)
Lead(II) sulphide + Oxygen -> Lead(II)Oxide + Sulphur(IV)oxide
PbS(s) + O2(g) -> Pb(s) + SO2(g)
Iron(II) sulphide + Oxygen -> Iron(II)Oxide + Sulphur(IV)oxide
FeS(s) + O2(g) -> Fe(s) + SO2(g)
Metallic ores that naturally occur as metallic carbonates are first heated in air.
They decompose/split to form the corresponding oxide and produce Carbon(IV)oxide gas.e.g.
Copper (II)carbonate -> Copper(II)oxide + Carbon(IV)oxide
CuCO3(s) -> CuO(s) + CO2(g)
Zinc (II)carbonate -> Zinc(II)oxide + Carbon(IV)oxide
ZnCO3(s) -> ZnO(s) + CO2(g)
Lead (II)carbonate -> Lead(II)oxide + Carbon(IV)oxide
PbCO3(s) -> PbO(s) + CO2(g)
Iron(II)carbonate -> Iron(II)oxide + Carbon(IV)oxide
FeCO3(s) -> FeO(s) + CO2(g)
Metallic ores
Water
Pure water is a colourless, odourless, tasteless,neutralliquid.
Pure water does not exist in nature but naturally in varying degree of purity.The main sources of water include rain, springs,borehole, lakes,seas and oceans:
Water is generally used for the following purposes:
(i)drinking by animals and plants.
(ii)washing clothes.
(iii)bleaching and dyeing.
(iv)generating hydroelectric power.
(v)cooling industrial processes.
Water dissolves many substances/solutes.
It is therefore called universal solvent.
It contains about 35% dissolved Oxygen which support aquatic fauna and flora. Water naturally exist in three phases/states solid ice,liquid water and gaseous water vapour.
The three states of water are naturally interconvertible.
The natural interconvertion of the three phases/states of water forms the water cycle.
Liquid water in land, lakes , seas and oceans use the solar/sun energy to evaporate/vapourize to form water vapour/gas. Solar/sun energy is also used during transpiration by plants and respiration by animals.During evaporation, the water vapour rises up the earths surface. Temperatures decrease with height above the earth surface increase. Water vapour therefore cools as it rises up.
At a height where it is cold enough to below 373Kelvin/100oC Water vapour looses enough energy to form tiny droplets of liquid.
The process by which a gas/water vapour changes to a liquid is called condensation/liquidification.
On further cooling, the liquid looses more energy to form ice/solid.
The process by which a liquid/water changes to a ice/solid is called freezing/solidification.
Minute/tiny ice/solid particles float in the atmosphere and coalesce/join together to form clouds.
When the clouds become too heavy they fall to the earths surface as rain/snow as the temperature increase with the fall.
Interconversion of the three phases/stateswater
Pure water has :(i) fixed/constant/sharp freezing point/melting point of 273K/0oC
(ii) fixed/constant/sharp boiling point of 373K/100oC at sea level/1 atmosphere pressure
(iii) fixed density of 1gcm-3
This is the criteria of identifying pure/purity of water.
Whether a substance is water can be determined by using the following methods:
a)To test for presence of water using anhydrous copper(II)suphate(VI)
Procedure.
Put about 2g of anhydrous copper(II)sulphate(VI)crystals into a clean test tube.Add three drops of tap water. Repeat the procedure using distilled water.
Observation.
Colour changes from white to blue
Explanation.
Anhydrous copper(II)sulphate(VI)is white. On adding water ,anhydrous copper(II)sulphate(VI) gains/reacts with water to form hydrated copper(II)sulphate(VI).
Hydrated copper(II)sulphate(VI) is blue.Hydrated copper(II)sulphate(VI) contain water of crystallization.
The change of white anhydrous copper(II)sulphate(VI) to bluehydrated copper(II)sulphate(VI) is a confirmatory test for the presence of water Chemical equation.
Anhydrous Hydrated
copper(II)sulphate(VI) + Water -> copper (II)sulphate(VI)
(white) (blue)
CuSO4(s) + 5H2O(l) ->CuSO4.5H2O(s)
b)To test for presence of water using anhydrous cobalt(II)chloride
Procedure.
Put about 5cm3 of water into a clean test tube.
Dip a dry anhydrous cobalt(II)chloride paper into the test tube.
Repeat the procedure using distilled water.
Observation.
Colour changes from blue to pink
Explanation.
Anhydrous cobalt(II)chloride is blue. On adding water,anhydrous cobalt(II)chloride gains/reacts with water to form hydrated cobalt(II)chloride.
Hydrated cobalt(II)chloride is pink.
Hydrated cobalt(II)chloride contain water of crystallization.
he change of blue anhydrous cobalt(II)chlorideto pink hydrated cobalt(II)chloride is a confirmatory test for the presence of water Chemical equation.
Anhydrous Hydrated cobalt(II)chloride + Water ->cobalt (II)chloride (Blue) (pink)
CoCl2(s) + 5H2O(l)->CoCl2.5H2O(s) Burning a candle in air
Most organic substances/fuels burn in air to produce water.Carbon(IV)oxide gas is also produced if the air is sufficient/excess.
Procedure
Put about 2g of anhydrous copper(II)sulphate(VI)crystals in a boiling tube.
Put about 5cm3 of lime water in a boiling tube.
Light a small candle stick.Place it below an inverted thistle/filter funnel Collect the products of the burning candle by setting the apparatus as below
Set up of apparatus
ObservationThe sunction pump pulls the products of burning into the inverted funnel. Colour of anhydrous copper(II) sulphate(VI)changes from white to blue.
A white precipitate is formed in the lime water/calcium hydroxide.
Explanation
When a candle burn it forms a water and carbon(IV)oxide.
Water turns anhydrous copper(II) sulphate(VI)changes from white to blue.
Carbon(IV)oxide gasforms white precipitatewhen bubbled in lime water/calcium hydroxide.
Since:
(i)hydrogen in the wax burn to form water
Hydrogen + Oxygen -> Water
(from candle) (from the air)
2H2(g) + O2(g) -> 2H2O (g/l)
(ii)carbon in the wax burn to form carbon(IV)oxide
Hydrogen + Oxygen -> Water
(from candle) (from the air)
C(s) + O2(g) -> CO2(g)
The candle before burning therefore contained only Carbon and Hydrogenonly.A compound made up of hydrogen and carbon is called Hydrocarbon.
A candle is a hydrocarbon.
Other hydrocarbons include: Petrol, diesel, Kerosene, and Laboratory gas.Hydrocarbons burn in air to form water and carbon(IV)oxide gas.
Hydrocarbons + Oxygen ->Water + Oxygen
Water pollution
Water pollution take place when undesirable substances are added into the water.Sources of water pollution include:
(i)Industrial chemicals being disposed into water bodies like rivers, lakes and oceans.
(ii)Dicharging untreated /raw sewage into water bodies.
(iii)Leaching of insecticides/herbicides form agricultural activities into water bodies.
(iv)Discharging non-biodegradable detergents after domestic and industrial use into water bodies.
(v)Petroleum oil spilling by ships and oil refineries
(vi)Toxic/poisonous gases from industries dissolving in rain .
(vii) Acidic gases from industries dissolving in rain to form “acid rain”
(viii)Discharging hot water into water bodies.This reduces the quantity of dissolved Oxygen in the water killing the aquatic fauna and flora.
Water pollution can be reduced by:
(i)reducing the use of agricultural fertilizers and chemicals in agricultural activities.
(ii)use of biological control method instead of insecticides and herbicides
(iii)using biodegradable detergents
Reaction of metals with water The higher the metal in the reactivity series the more reactive the metal with water.
The following experiments shows the reaction of metals with cold water and water vapour/steam.
(a)Reaction of sodium/ potassium with cold water:
Procedure
Put about 500cm3 of water in a beaker.Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
Cut a very small piece of sodium .Using a pair of forceps, put the metal into the water.
Observation
Sodium melts to a silvery ball that floats and darts on the surface decreasing in size.Effervescence/fizzing/ bubbles of colourless gas produced.
Colour of phenolphthalein turns pink
Colour of litmus solution turns blue
Colour of methy orange solution turns Orange
Colour of universal indicator solution turns blue
Explanation
Sodium is less dense than water.Sodium floats on water and vigorously react to form an alkaline solution of sodium hydroxide and producing hydrogen gas.
Sodium is thus stored in paraffin to prevent contact with water.
Chemical equation
Sodium + Water -> Sodium hydroxide + Hydrogen gas
2Na(s) + 2H2O(l) -> 2NaOH(aq) + H2(g)
To collect hydrogen gas , Sodium metal is forced to sink to the bottom of the trough/beaker by wrapping it in wire gauze/mesh.
burning a candle Potassium is more reactive than Sodium. On contact with water it explodes/burst into flames.An alkaline solution of potassium hydroxide is formed and hydrogen gas
Chemical equation
Potassium + Water -> Potassium hydroxide + Hydrogen gas
2K(s) + 2H2O(l) -> 2KOH(aq) + H2(g)
Caution: Reaction of Potassium with water is very risky to try in a school laboratory.
(b)Reaction of Lithium/ Calcium with cold water:
Procedure
Put about 200cm3 of water in a beaker.Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
Cut a small piece of Lithium .
Using a pair of forceps, put the metal into the water. Repeat with a piece Calcium metal
Observation
Lithium sinks to the bottom of the water.Rapid effervescence/fizzing/ bubbles of colourless gas produced.
Colour of phenolphthalein turns pink
Colour of litmus solution turns blue
Colour of methy orange solution turns Orange
Colour of universal indicator solution turns blue
Explanation
Lithium and calcium are denser than water. Both sink in water and vigorously react to form an alkaline solution of Lithium hydroxide / calcium hydroxide and producing hydrogen gas.
Lithium is more reactive than calcium.
It is also stored in paraffin like Sodium to prevent contact with water.
Chemical equation
Lithium + Water -> Lithium hydroxide + Hydrogen gas
2Li(s) + 2H2O(l) -> 2LiOH(aq) + H22(g) Calcium + Water -> Calcium hydroxide + Hydrogen gas Ca(s) + 2H2O(l) -> Ca(OH)2(aq) + H2(g)
(c)Reaction of Magnesium/Zinc/ Iron with Steam/water vapour:Procedure method 1
Place some wet sand or cotton/glass wool soaked in water at the bottom of an ignition/hard glass boiling tube.
Polish magnesium ribbon using sand paper.
Coil it at the centre of the ignition/hard glass boiling tube.
Set up the apparatus as below.
Heat the wet sand or cotton/glass wool soaked in water gently to:
(i)drive away air in the ignition/hard glass boiling tube.
(ii)generate steam
Heat the coiled ribbon strongly using another burner.Repeat the experiment using Zinc powder and fresh Iron filings.
Set up of apparatus
Observations
(i)With Magnesium ribbon:
The Magnesium glow with a bright flame (and continues to burn even if heating is stopped) White solid /ash formed
White solid /ash formed dissolve in water to form a colourless solution
Colourless gas produced/collected that extinguish burning splint with “pop sound”(ii)With Zinc powder:
The Zinc powder turns red hot on strong heating
Yellow solid formed that turn white on cooling
White solid formed on cooling does not dissolve in water.
(iii)With Iron fillings:
The Iron fillings turns red hot on strong heating
Dark blue solid formed
Dark blue solid formed does not dissolve in water.
Procedure method 2
Put some water in a round bottomed flask
Polish magnesium ribbon using sand paper.
Coil it at the centre of a hard glass tube
Set up the apparatus as below.
Heat water strongly to boil so as to:
(i)drive away air in the glass tube.
(ii)generate steam
Heat the coiled ribbon strongly using another burner. Repeat the experiment using Zinc powder and fresh Iron filings.
Method 2: Reaction of Steam With Magnesium Zinc/ironObservations
(i)With Magnesium ribbon:
The Magnesium glow with a bright flame (and continues to burn even if heating is stopped) White solid /ash formed
White solid /ash formed dissolve in water to form a colourless solution
Colourless gas produced/collected that extinguish burning splint with “pop sound”(ii)With Zinc powder:
The Zinc powder turns red hot on strong heating
Yellow solid formed that turn white on cooling
White solid formed on cooling does not dissolve in water.
(iii)With Iron fillings:
The Iron fillings turns red hot on strong heating
Dark blue solid formed
Dark blue solid formed does not dissolve in water.
Explanations
(a)Hot magnesium burn vigorously in steam. The reaction is highly exothermic generating enough heat/energy to proceed without further heating.
White Magnesium oxide solid/ash is left as residue.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Magnesium + Steam ->Magnesium oxide + Hydrogen
Mg(s) + H2O(g) -> MgO(s) + H2(g)
Magnesium oxide reacts /dissolves in water to form an alkaline solution of Magnesium oxide
Chemical Equation
Magnesium oxide + Water ->Magnesium hydroxide MgO(s) + H2O(l) -> Mg(OH) 2 (aq) (b)Hot Zinc react vigorously in steam forming yellow Zinc oxide solid/ash as residue which cools to white.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Zinc + Steam -> Zinc oxide + Hydrogen Zn(s) + H2O(g) ->ZnO(s) + H2(g)
Zinc oxide does not dissolve in water.
(c)Hot Iron react with steam forming dark blue tri iron tetra oxide solid/ash as residue.
Hydrogen gas is produced .It extinguishes a burning splint with a “pop sound”.
Chemical Equation Iron + Steam -> Tri iron tetra oxide + Hydrogen 2Fe(s) + 4H2O(g) -> Fe2O4(s) + 4H2(g) Tri iron tetra oxide does not dissolve in water.
(d)Aluminium reacts with steam forming an insoluble coat/cover of impervious layer of aluminium oxide on the surface preventing further reaction.
(e) Lead, Copper, Mercury, Silver, Gold and Platinum do not react with either water or steam.
Hydrogen
Occurrence
Hydrogen does not occur free in nature. It occurs as Water and in Petroleum.
School laboratory Preparation
Procedure
Put Zinc granules in a round/flat/conical flask. Add dilute sulphuric(VI) /Hydrochloric acid. Add about 3cm3 of copper(II)sulphate(VI) solution.
Collect the gas produced over water as in the set up below.
Discard the first gas jar. Collect several gas jar.
Observation/ExplanationZinc reacts with dilute sulphuric(VI)/hydrochloric acid to form a salt and produce hydrogen gas.
When the acid comes into contact with the metal,there is rapid effervescence/ bubbles /fizzing are produced and a colourless gas is produced that is collected:
(i) over water because it is insoluble in water
(ii)through downward displacement of air/upward delivery because it is less dense than air.
The first gas jar is impure. It contains air that was present in the apparatus.
Copper(II)sulphate(VI)solution act as catalyst.
Chemical equation
(a) Zinc + Hydrochloric acid ->Zinc chloride + Hydrogen
Zn(s) + 2HCl(aq) ->ZnCl2(aq) + H2(g)
Ionic equation
Zn(s) + 2H+(aq) -> Zn2+(aq) + H2(g)
Zinc + Sulphuric(VI)acid ->Zinc Sulphate(VI)+ Hydrogen
Zn(s) + H2SO4(aq) -> ZnSO4(aq)
+ H2(g) Ionic equation
Zn (s) + 2H+(aq) -> Zn2+(aq) + H2(g)
(b)Chemical equation
Magnesium + Hydrochloric acid -> Magnesium chloride + Hydrogen
Mg(s) + 2HCl(aq) -> MgCl2(aq)
+ H2(g)
Ionic equation
Mg (s) + 2H+(aq) -> Mg2+(aq) + H2(g)
Magnesium+ Sulphuric(VI)acid ->Magnesium Sulphate(VI)+ Hydrogen Mg(s) + H2SO4(aq) -> MgSO2(aq) + H2(g)
Ionic equation
Mg (s) + 2H+(aq) -> Mg2+(aq) + H2(g)
(c)Chemical equation
Iron + Hydrochloric acid -> Iron(II)chloride + Hydrogen
Fe(s) + 2HCl(aq) -> FeCl2(aq) + H2(g)
Ionic equation
Fe (s) + 2H+(aq) ->Fe2+(aq) + H2(g)
Iron+ Sulphuric(VI)acid ->Iron(II) Sulphate(VI)+ Hydrogen
Fe(s) + H2SO4(aq)->FeSO4(aq) + H2(g)
Ionic equation
Fe (s) + 2H+(aq) ->Fe2+(aq) + H2(g)
Note
1.Hydrogen cannot be prepared from reaction of:
(i)Nitric(V)acid and a metal. Nitric(V)acid is a strong oxidizing agent. It oxidizes hydrogen gas to water.
(ii)dilute sulphuric(VI)acid with calcium/Barium/Lead because Calcium sulphate(VI),Barium sulphate(VI) and Lead(II)sulphate(VI) salts formed are insoluble.
Once formed, they cover/coat the unreacted calcium/Barium/Lead stopping further reaction and producing very small amount/volume of hydrogen gas.
(iii)dilute acid with sodium/potassium. The reaction is explosive.
Properties of Hydrogen gas
(a)Physical properties
1. Hydrogen is a neutral ,colourless and odourless gas.
When mixed with air it has a characteristic pungent choking smell
2. It is insoluble in water thus can be collected over water.
3. It is the lightest known gas. It can be transferred by inverting one gas jar over another.
(b)Chemical properties.
(i)Burning
I. Hydrogen does not support burning/combustion.When a burning splint is inserted into a gas jar containing Hydrogen,the flame is extinguished/put off.
II.Pure dry hydrogen burn with a blue quiet flame to form water. When a stream of pure dry hydrogen is ignited, it catches fire and continues to burn with a blue flame.
III.Impure(air mixed with) hydrogen burns with an explosion.Small amount/volume of air mixed with hydrogen in a test tube produce a small explosion as a “pop” sound.
This is the confirmatory test for the presence of Hydrogen gas. A gas that burns with a “pop” sound is confirmed to be Hydrogen.
(ii)Redox in terms of Hydrogen transfer
Redox can also be defined in terms of Hydrogen transfer.
(i)Oxidation is removal of Hydrogen
(ii)Reduction is addition of Hydrogen
(iii)Redox is simultaneous addition and removal of Hydrogen
Example
When a stream of dry hydrogen gas is passed through black copper (II) oxide, hydrogen gas gains the oxygen from copper(II)oxide.
Black copper (II) oxide is reduced to brown copper metal.
Black copper(II)oxide os thus the Oxidizing agent.
Hydrogen gas is oxidized to Water. Hydrogen is the Reducing agent.
Set up of apparatus
(a)Chemical equation
(i)In glass tube Copper(II)Oxide + Hydrogen ->Copper + Hydrogen gas
(oxidizing agent) (reducing agent)
(black) (brown)
CuO (s) + H2(g) -> Cu(s) + H2O(l)
(ii)when excess Hydrogen is burning.
Oxygen + Hydrogen -> Water
O2(g) + 2H2(g) ->2H2O(l)
(b)Chemical equation
(i)In glass tube
Lead(II)Oxide + Hydrogen ->Lead + Hydrogen gas
(oxidizing agent) (reducing agent)
(brown when hot/ (grey)
yellow when cool)
PbO (s) + H2(g) ->Pb(s) + H2O(l)
(ii)when excess Hydrogen is burning.
Oxygen + Hydrogen -> Water
O2(g) + 2H2(g) -> 2H2O(l)
(c)Chemical equation
(i)In glass tube
Iron(III)Oxide + Hydrogen ->Iron + Hydrogen gas
(oxidizing agent)
(reducing agent)
(Dark grey)
(grey)
Fe2O3 (s) + 3H2(g) ->Fe(s) + 3H2O(l)
(ii)when excess Hydrogen is burning.
Oxygen + Hydrogen -> Water
O2(g) + 2H2(g) -> 2H2O(l)
(iii)Water as an Oxide as Hydrogen
Burning is a reaction of an element with Oxygen.
The substance formed when an element burn in air is the oxide of the element.
When hydrogen burns, it reacts/combines with Oxygen to form the oxide of Hydrogen.
The oxide of Hydrogen is called water.
Hydrogen is first dried because a mixture of Hydrogen and air explode.
The gas is then ignited .
The products condense on a cold surface/flask containing a freezing mixture.
A freezing mixture is a mixture of water and ice.
The condensed products are collected in a receiver as a colourless liquid.
Tests
(a) When about 1g of white anhydrous copper(II)sulphate(VI)is added to a sample of the liquid ,it turns to blue. This confirms the liquid formed is water.
(b) When blue anhydrous cobalt (II)chloride paper is dipped in a sample of the liquid ,it turns to pink. This confirms the liquid formed is water.
(c)When the liquid is heated to boil, its boiling point is 100oC at sea level/one atmosphere pressure.This confirms the liquid is pure water.
Uses of Hydrogen gas
1. Hydrogenation/Hardening of unsaturated vegetable oils to saturated fats/margarine.
When Hydrogen is passed through unsaturated compounds in presence of Nickel catalyst and about 150oC, they become saturated.
Most vegetable oil are unsaturated liquids at room temperature. They become saturated and hard through hydrogenation.
2. In weather forecast balloons.
Hydrogen is the lightest known gas. Meteorological data is collected for analysis by sending hydrogen filled weather balloons to the atmosphere.
The data collected is then used to forecast weather conditions.
3.In the Haber process for the manufacture of Ammonia
Hydrogen is mixed with Nitrogen in presence of Iron catalyst to form Ammonia gas.
Ammonia gas is a very important raw material for manufacture of agricultural fertilizers.
4.In the manufacture of Hydrochloric acid.
Limited volume/amount of Hydrogen is burnt in excess chlorine gas to form Hydrogen chloride gas.
Hydrogen chloride gas is dissolved in water to form Hydrochloric acid.
Hydrochloric acid is used in pickling/washing metal surfaces.
5.As rocket fuel.
Fixed proportions of Hydrogen and Oxygen when ignited explode violently producing a lot of energy/heat.This energy is used to power/propel a rocket to space.
6.In oxy-hydrogen flame for welding.
A cylinder containing Hydrogen when ignited in pure Oxygen from a second cylinder produces a flame that is very hot. It is used to cut metals and welding.
Sample revision questions
1.A colourless liquid was added anhydrous copper(II)sulphate(VI) which turned blue.
(a)Why is it wrong to conclude the liquid was pure water?
Anhydrous copper(II)sulphate(VI) test for presence of water.Purity of water is determined from freezing/melting/boiling point.
(b)Write an equation for the reaction that take place with anhydrous copper(II)sulphate(VI)
Anhydrous copper(II)sulphate(VI) + Water ->hydrated copper(II)sulphate(VI) CuSO4(s) + 5H2O(l) ->CuSO4.5H2O(s)
(c)(i)Which other compound would achieve the same results as anhydrous copper(II)sulphate(VI)
Anhydrous cobalt (II)chloride/CoCl2.6H2O
(ii)Write the equation for the reaction
Anhydrous cobalt (II)chloride + Water ->hydratedcobalt (II)chloride
CoCl2 (s) + 6H2O(l) -> CoCl2.6H2O (s)
(d)Complete the equation
(i) Sulphur(VI)oxide + Water ->Sulphuric(VI)acid
(ii) Sulphur(IV)oxide + Water ->Sulphuric(IV)acid
(iii) Carbon(IV)oxide + Water ->Carbonic(IV)acid
(iv) Nitrogen(IV)oxide + Water ->Nitric(V)acid
(v) Phosphorus(V)oxide + Water ->Phosphoric(V)acid
(vi) Sodium oxide + Water ->Sodium hydroxide
(vi) Sodium peroxide + Water ->Sodium hydroxide
2. Metal B reacts with steam. Metal C reacts with cold water.Metal A does not react with water.
(a)Arrange the metals as they should appear in the reactivity series.
B
C
A
(b)Aproduct residue in Dwhich was brown when hot but turned yellow on cooling during the reaction of metal B was formed. Gas E was also evolved.Identify
(i)Metal B Lead/Pb
(ii)Residue D Lead(II)oxide/PbO
(iii)Gas E Hydrogen/H2
(c)A portion of product residue in D was added dilute nitric(V)acid.
Another portion of product residue in D was added dilute sulphuric(VI)acid.State and explain the observations made.
When added dilute nitric(V)acid, D dissolves to form a colourless solution.
Lead(II)Oxide +dilute nitric(V)acid -> Lead(II) nitrate(V)+ Water
PbO (s) + 2HNO3(aq)-> Pb(NO3)2(aq) + H2O(l)
When added dilute sulphuric(VI)acid, D does not dissolve.
A white suspension/precipitate was formed.Lead(II)Oxide reacts withsulphuric(VI)acid to form insolubleLead(II)sulphate(VI) that cover/coat unreacted Lead(II)Oxide, stopping further reaction.
Lead(II)Oxide +dilute sulphuric(VI)acid -> Lead(II) sulphate(VI) + Water PbO (s) + H2SO4(aq) ->PbSO4 (s) + H2O(l)
3. (a) Hydrogen can reduce copper(II)Oxide but not alluminium oxide. Explain
(b) When water reacts with potassium metal the hydrogen produced ignites explosively on the surface of water.
(i) What causes this ignition?
(ii) Write an equation to show how this ignition occurs
2. In an experiment, dry hydrogen gas was passed over hot copper (II) oxide in a combustion tube as shown in the diagram below:
(a) Complete the diagram to show how the other product, substance R could be collected in the laboratory.
(b) Describe how copper could be obtained from the mixture containing copper (II) oxide
3. The setup below was used to investigate the reaction between metals and
(a) Identify solid X and state its purposeSolid X .…………………………………………………………………..
Purpose …………………………………………………………………..
(b) Write a chemical equation for the reaction that produces the flame.
4. Gas P was passed over heated magnesium ribbon and hydrogen gas was collected as showing the diagram below:
(i) Name gas P…………………………………………………………………………………………………
(ii) Write an equation of the reaction that takes place in the combustion tube
(iii) State one precaution necessary at the end of this experiment
5. When hydrogen is burnt and the product cooled, the following results are obtained as shown in the diagram below:
(a) Write the equation for the formation of liquid Y(b) Give a chemical test for liquid Y
6. Jane set-up the experiment as shown below to collect a gas. The wet sand was heated
(a) Complete the diagram for the laboratory preparation of the gas(b) Why was it necessary to heat wet sand before heating Zinc granules?
7.
(a) Between N and M which part should be heated first? Explain(b) Write a chemical equation for the reaction occurring in the combustion tube.
8. The set-up below was used to investigate electrolysis of a certain molten compound;
(a) Complete the circuit by drawing the cell in the gap left in the diagram(b) Write half-cell equation to show what happens at the cathode
(c) Using an arrow show the direction of electron flow in the diagram above
9. Hydrogen can be prepared by reacting zinc with dilute hydrochloric acid.
a) Write an equation for the reaction.
b) Name an appropriate drying agent for hydrogen gas.
c) Explain why copper metal cannot be used to prepare hydrogen gas.
d) Hydrogen burns in oxygen to form an oxide.
(i) Write an equation for the reaction.
(ii) State two precautions that must be taken before the combustion begins and at the end of the combustion.
e) Give two uses of hydrogen gas.
f) When zinc is heated to redness in a current of steam, hydrogen gas is obtained. Write an equation for the reaction.
g) Element Q reacts with dilute acids but not with cold water. Element R does not react with dilute acids.
Elements S displaces element P from its oxide. P reacts with cold water.
Arrange the four elements in order of their reactivity, starting with the most reactive.
h) Explain how hydrogen is used in the manufacture of margarine.
10. a) The set-up below is used to investigate the properties of hydrogen.
i) On the diagram, indicate what should be done for the reaction to occurii) Hydrogen gas is allowed to pass through the tube for some time before it is lit. Explain
iii) Write an equation for the reaction that occurs in the combustion tube
iv) When the reaction is complete, hydrogen gas is passed through the apparatus until they cool down . Explain
v) What property of hydrogen is being investigated?
vi) What observation confirms the property stated in (v) above?
vii) Why is zinc oxide not used to investigate this property of hydrogen gas?
11. The set up below was used to collect gas K, produced by the reaction between water
(a) Name gas K ……………………………………………………………..(b) At the end of the experiment, the solution in the beaker was found to be a weak base. Explain
why the solution is a weak base.
FREE CHEMISTRY NOTES FOR FORM ONE TO FOUR.
FREE LATEST CHEMISTRY NOTES FOR FORM 2
FREE LATEST CHEMISTRY NOTES FOR FORM 1
FREE LATEST CHEMISTRY NOTES FOR FORM 1 UPDATED
FREE LATEST CHEMISTRY NOTES FOR FORM
FREE LATEST CHEMISTRY NOTES FOR FORM 2 UPDATED
FREE LATEST CHEMISTRY NOTES FOR FORM 1 NEW
FREE LATEST CHEMISTRY NOTES FOR FORM 1-4
FREE LATEST CHEMISTRY NOTES FOR FORM 1 UPDATED COPY
FREE LATEST CHEMISTRY NOTES FOR FORM 1-4 BOOKLET
FREE LATEST CHEMISTRY NOTES FOR FORM 1-4 UPDATED
FREE LATEST CHEMISTRY NOTES FOR FORM PRACTICALS GUIDE
FREE LATEST CHEMISTRY NOTES FOR FORM 3
FREE LATEST CHEMISTRY NOTES FOR FORM ONE TO FOUR

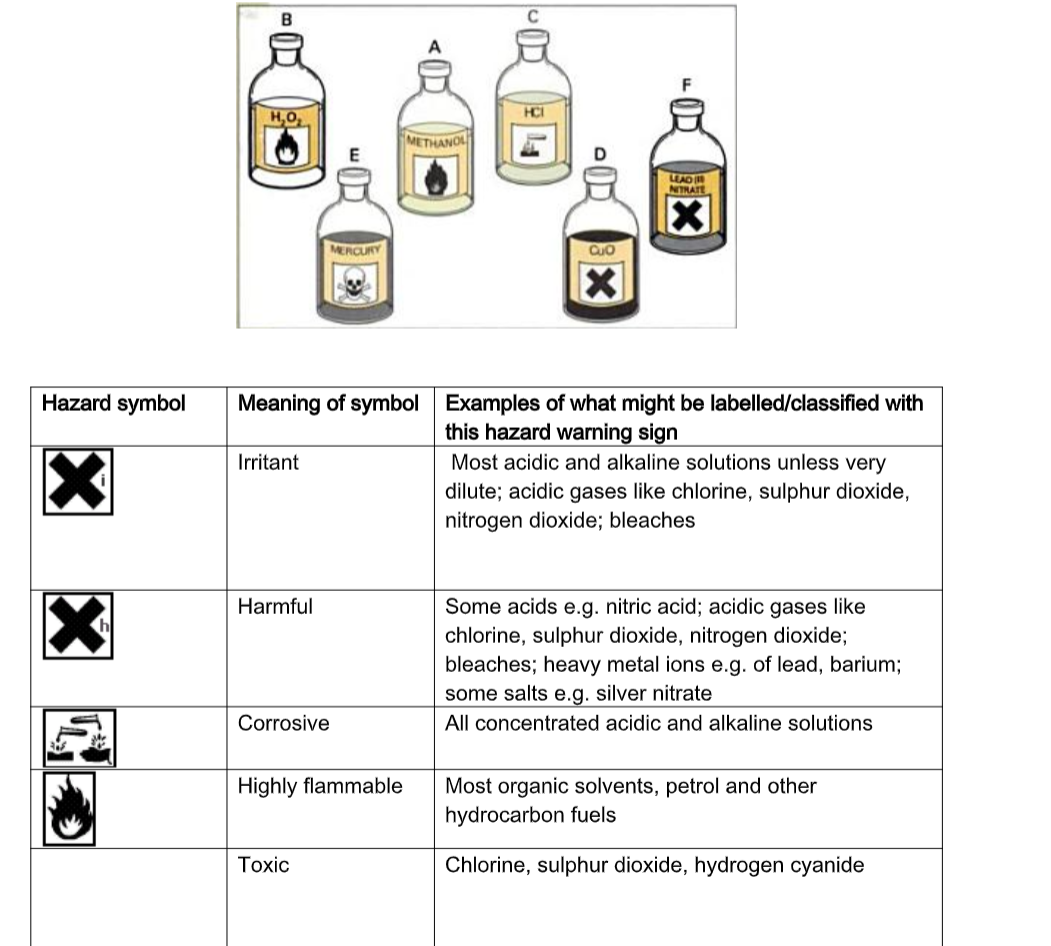
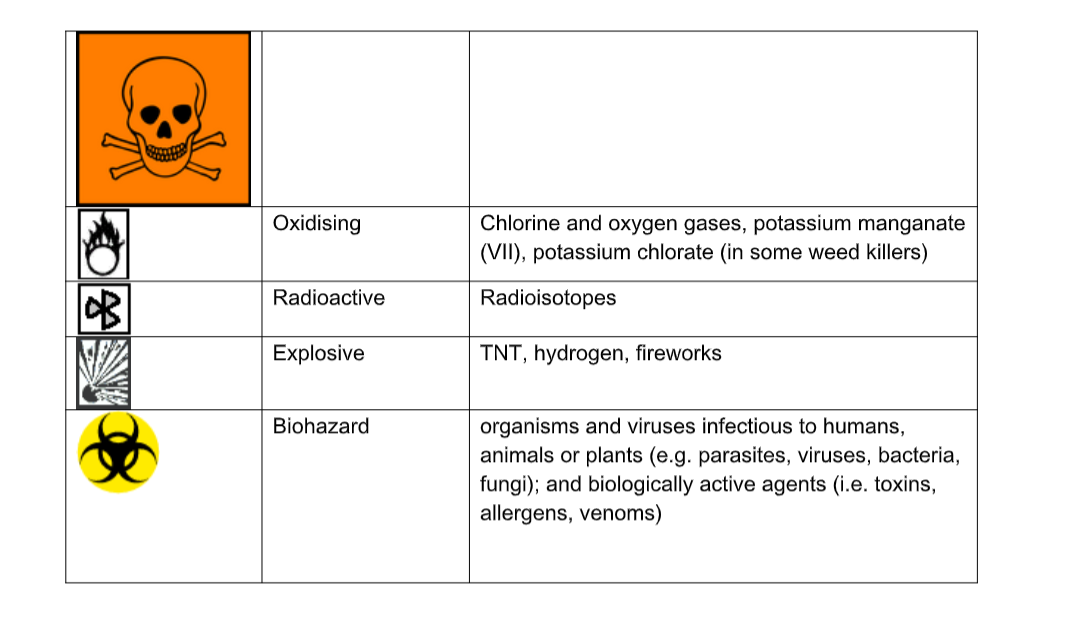
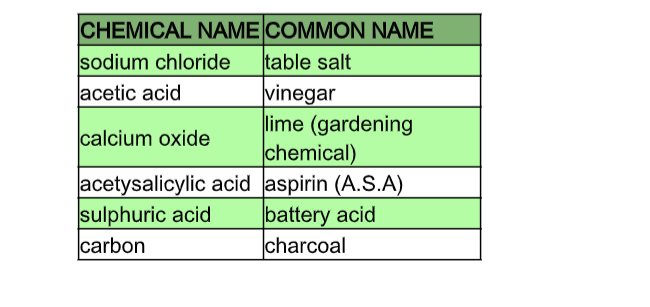
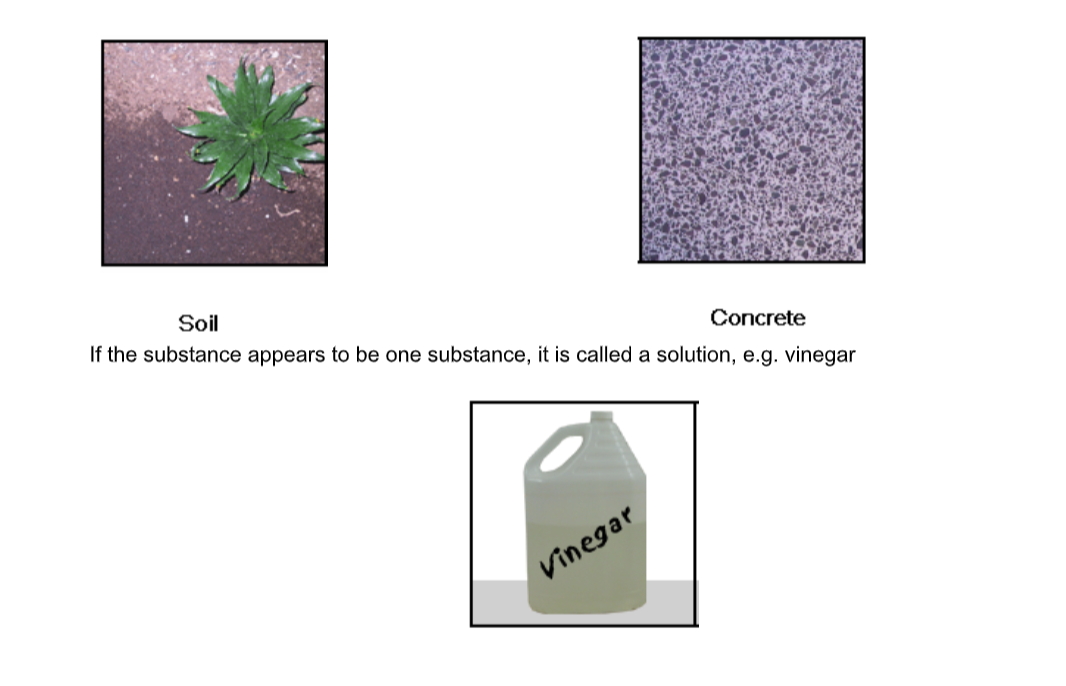


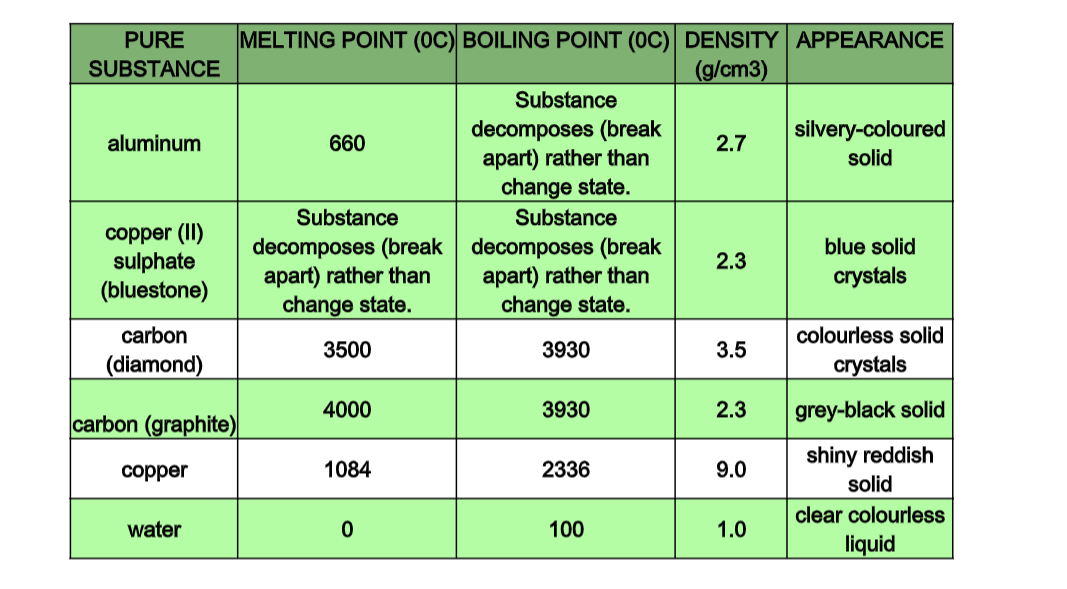
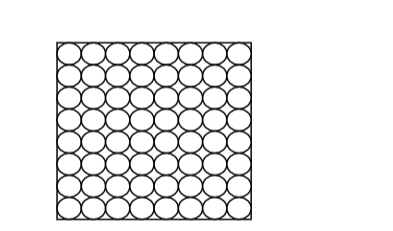
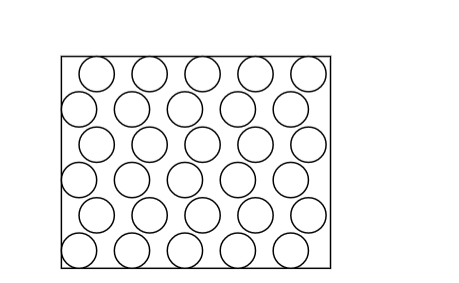
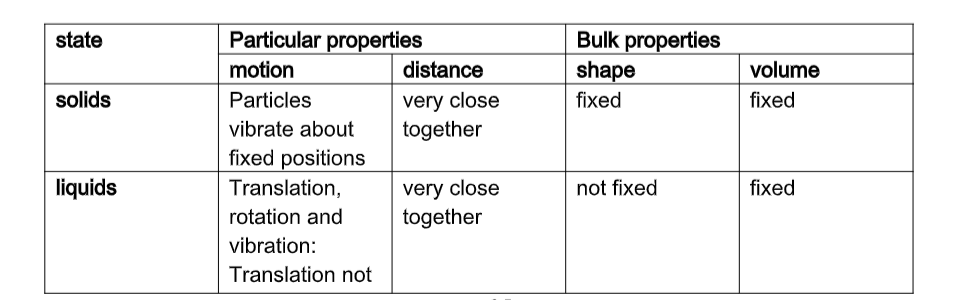
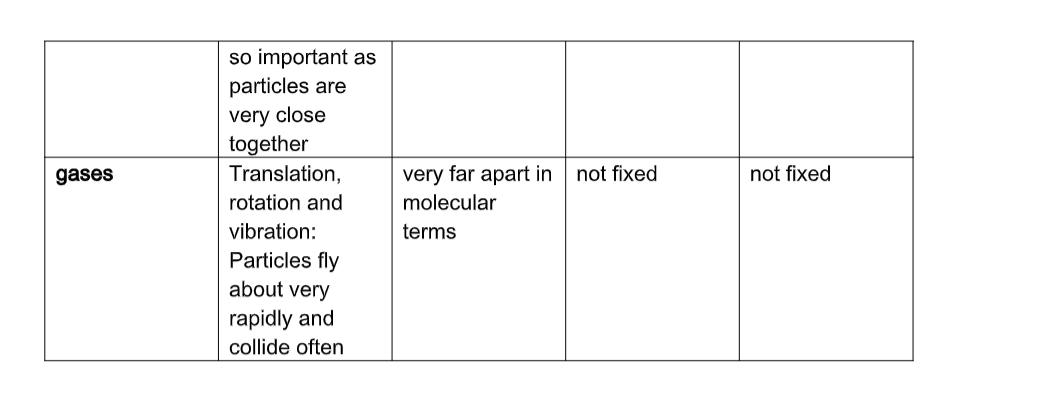
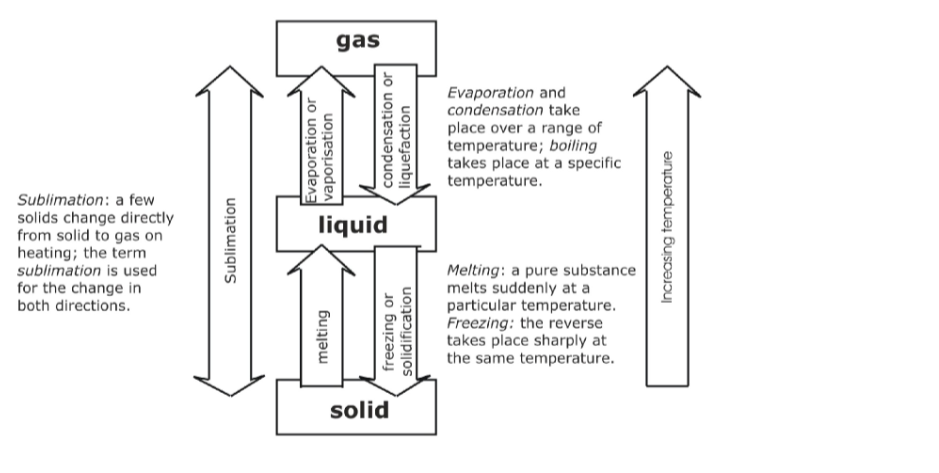
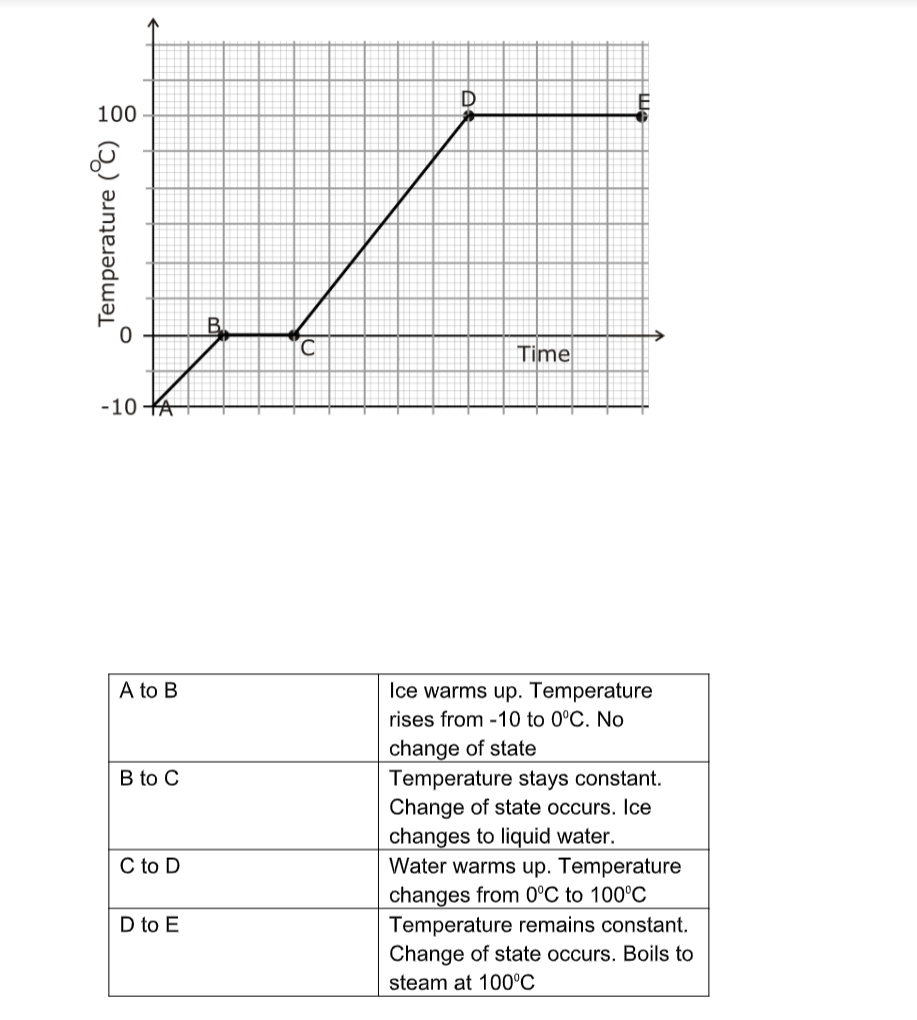
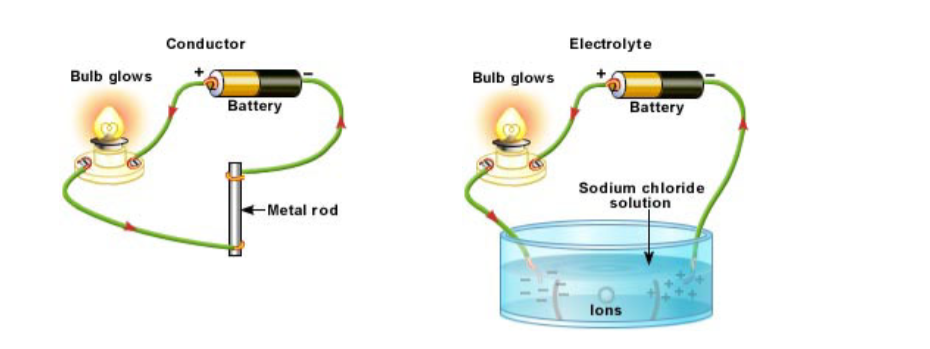

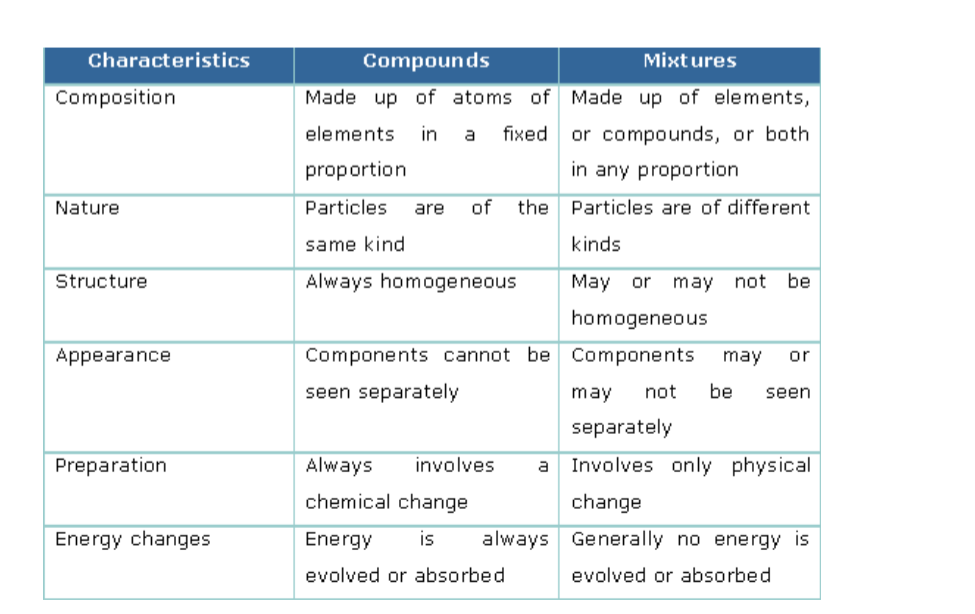
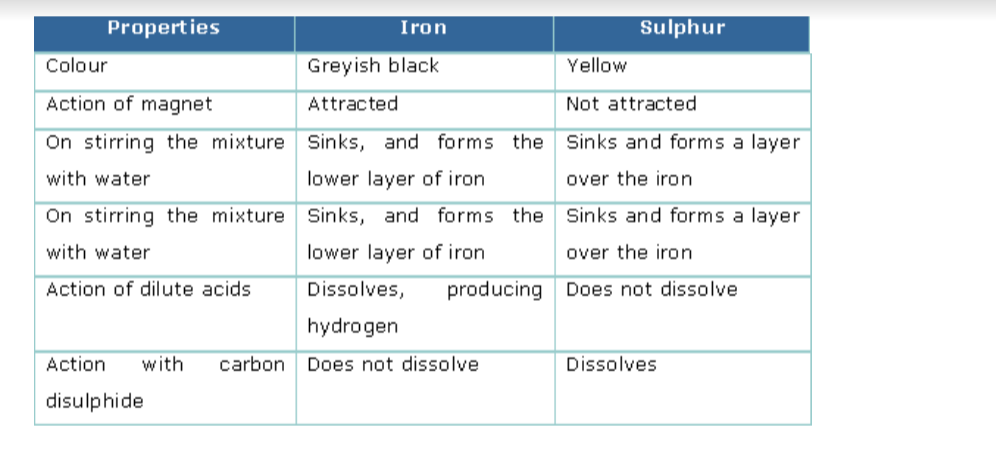
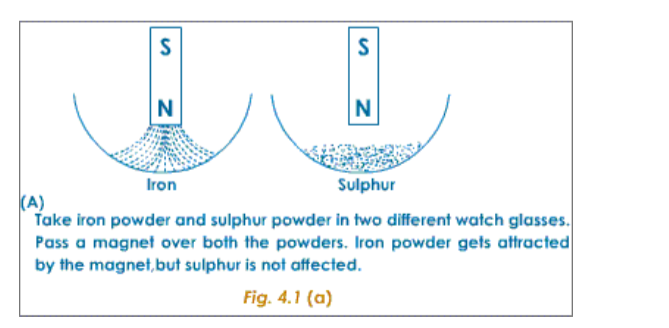
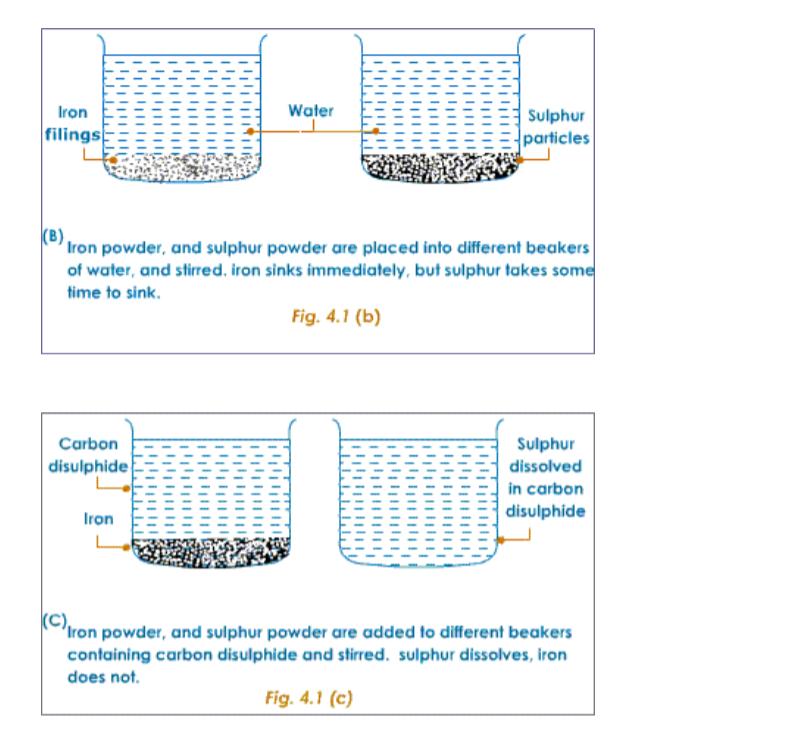
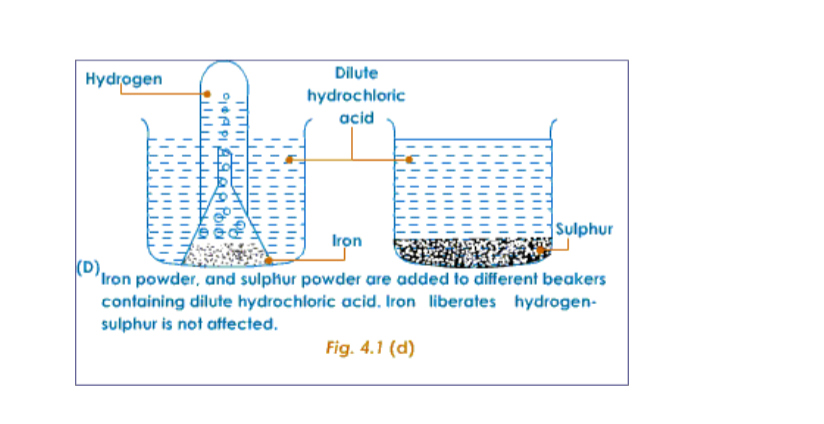
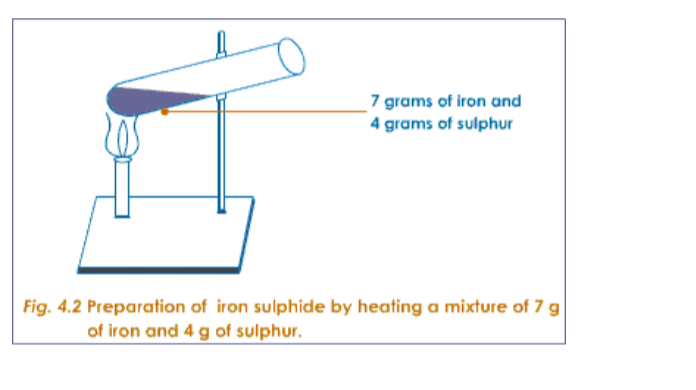
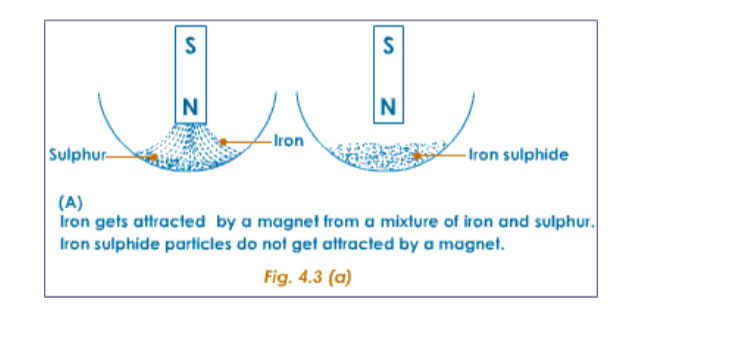
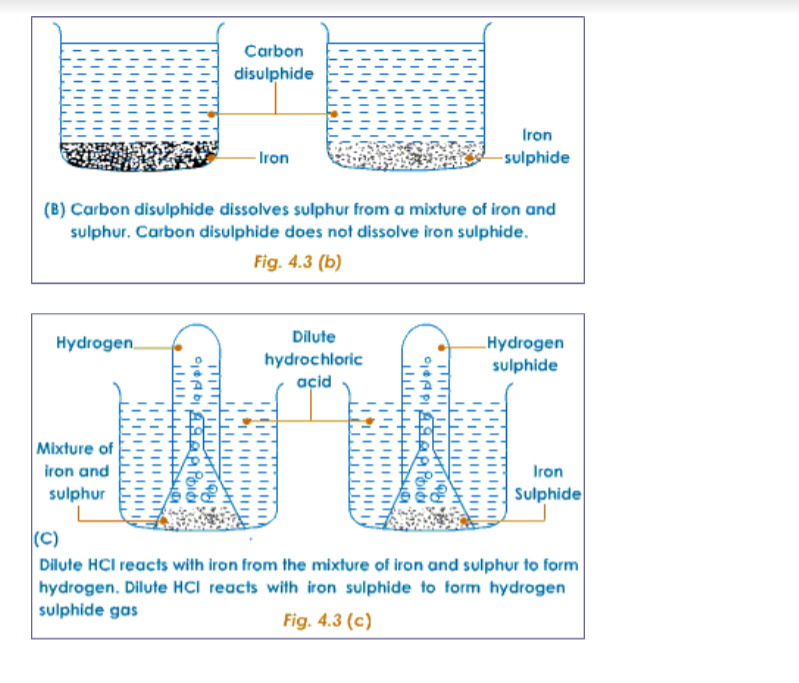
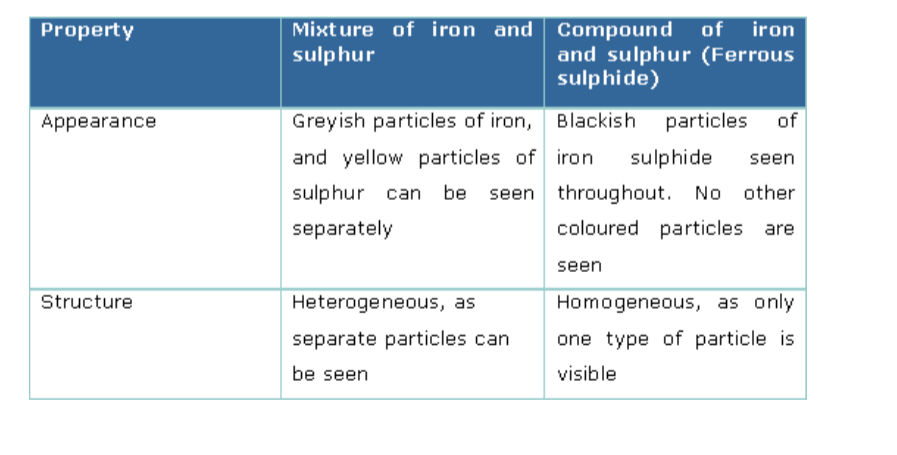
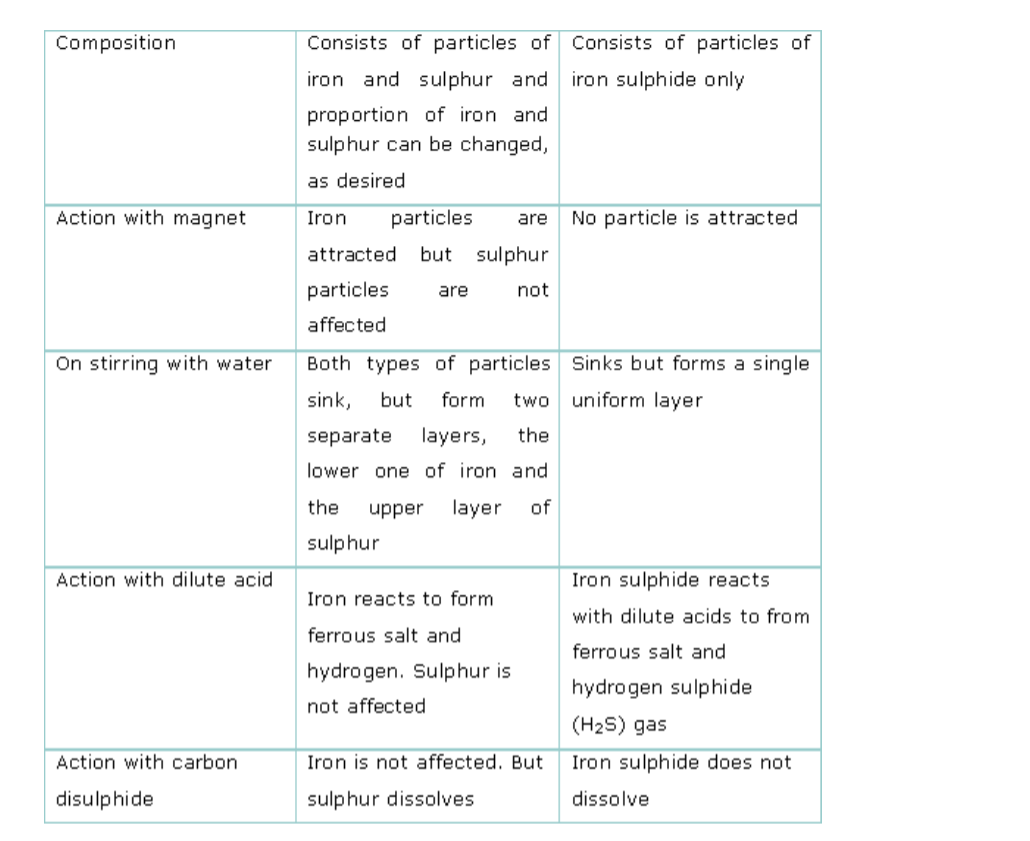
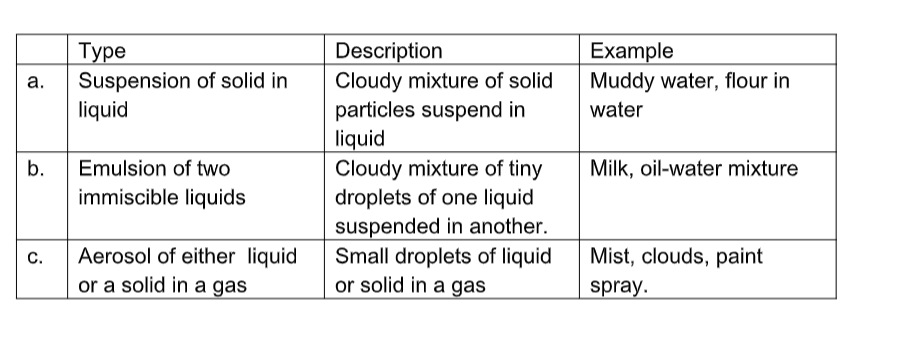
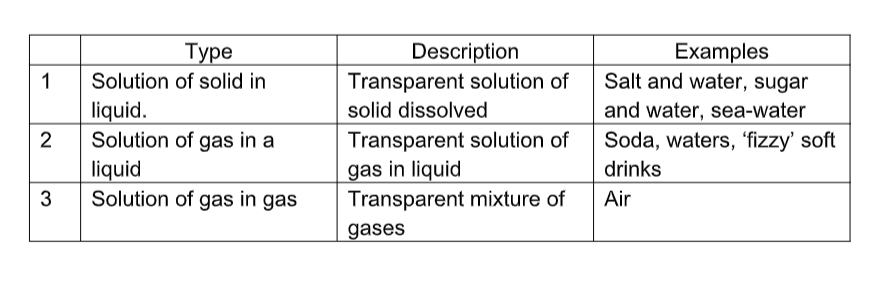
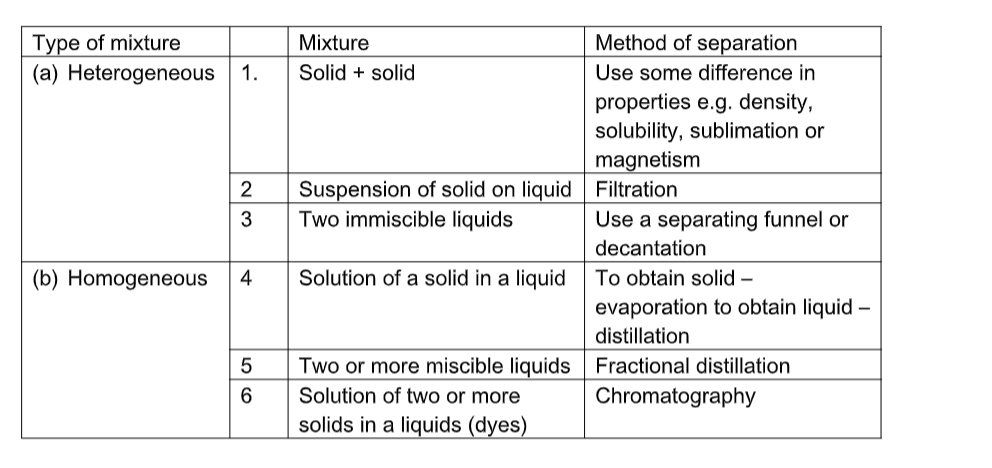
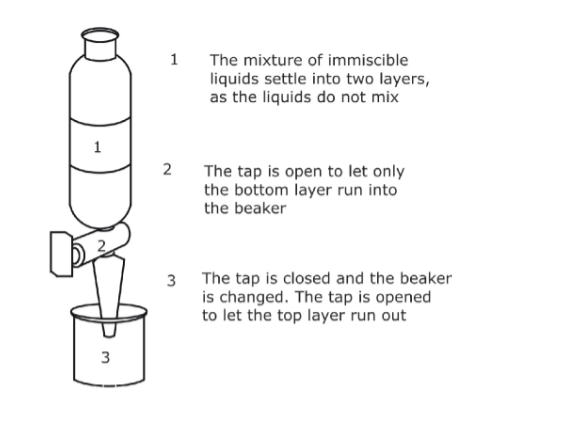
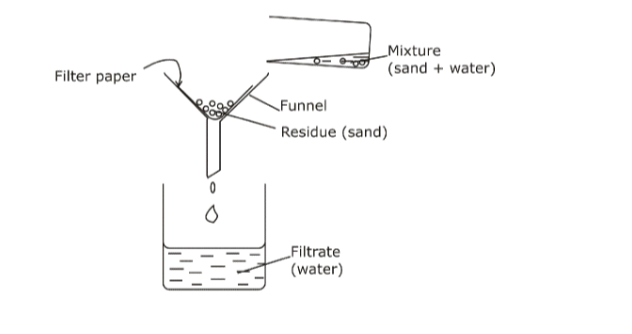
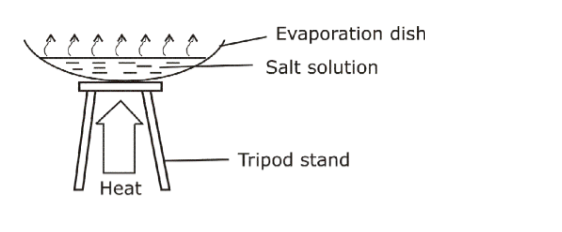
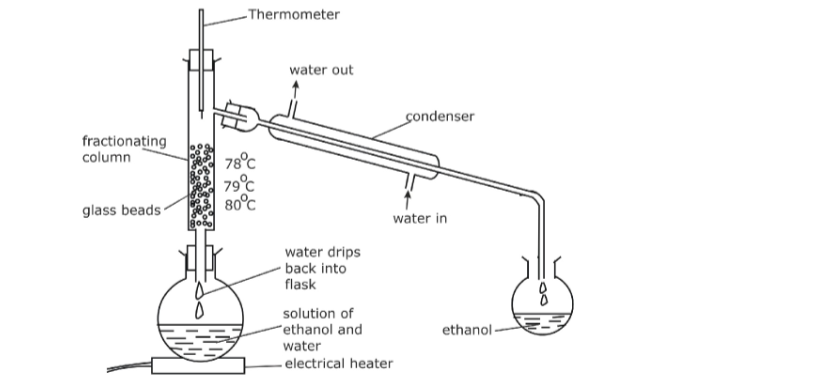
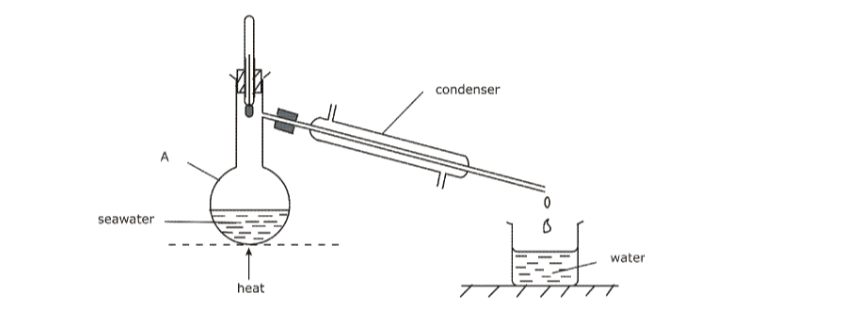
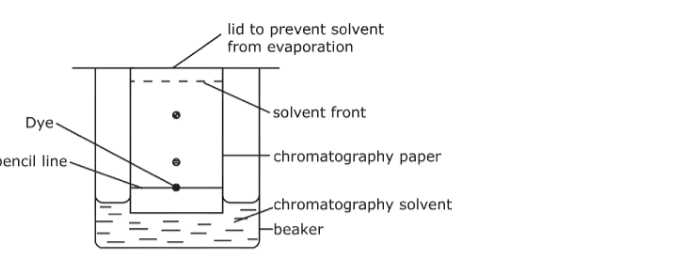
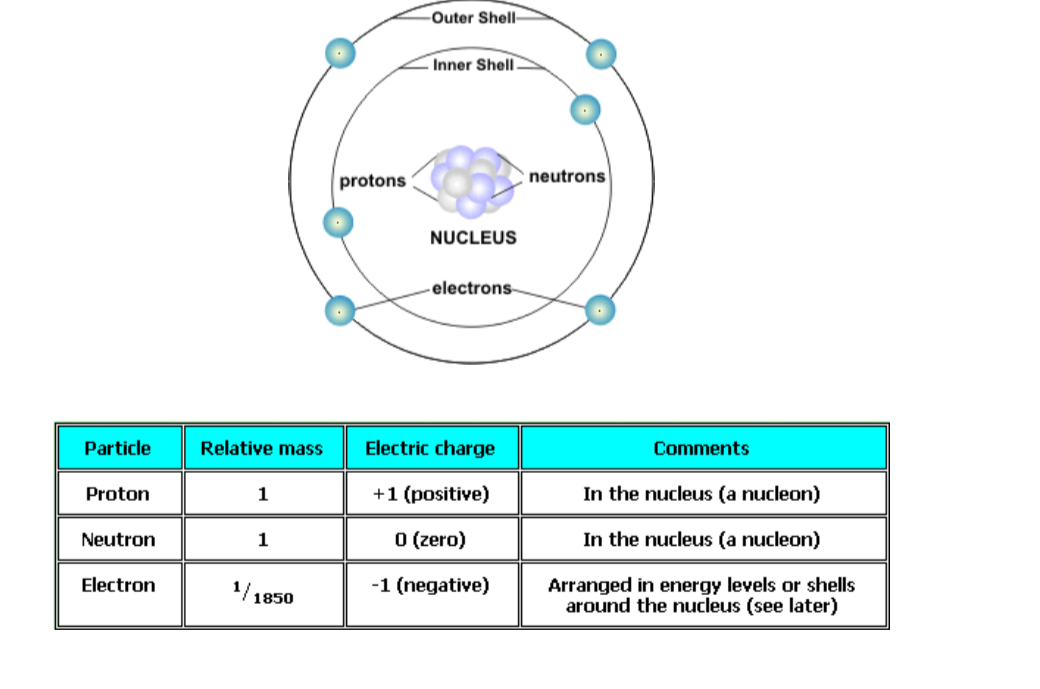
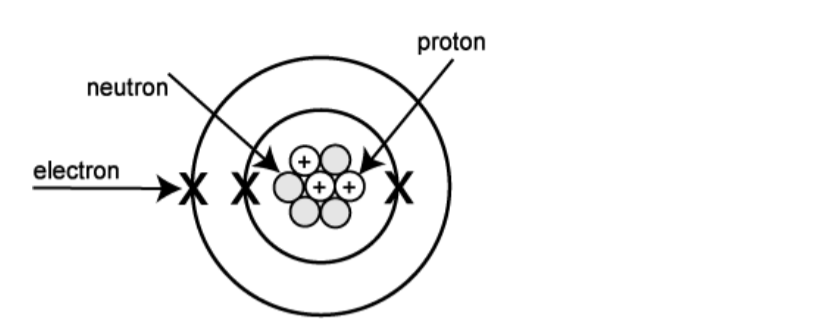
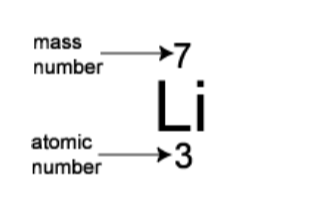
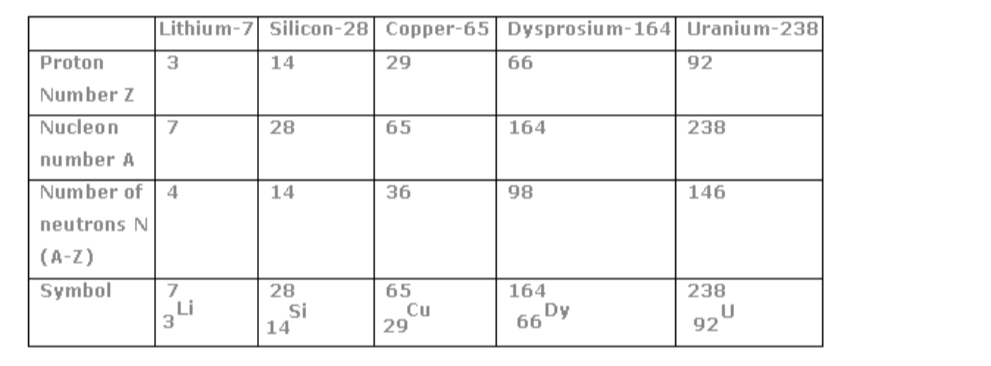
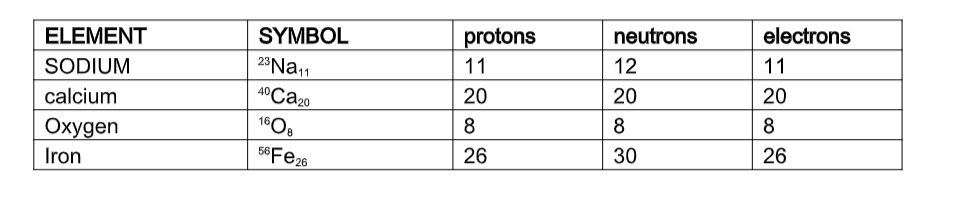
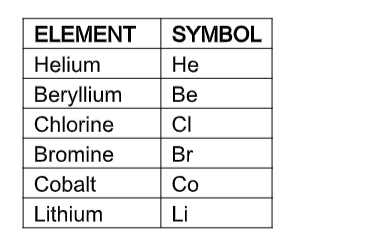
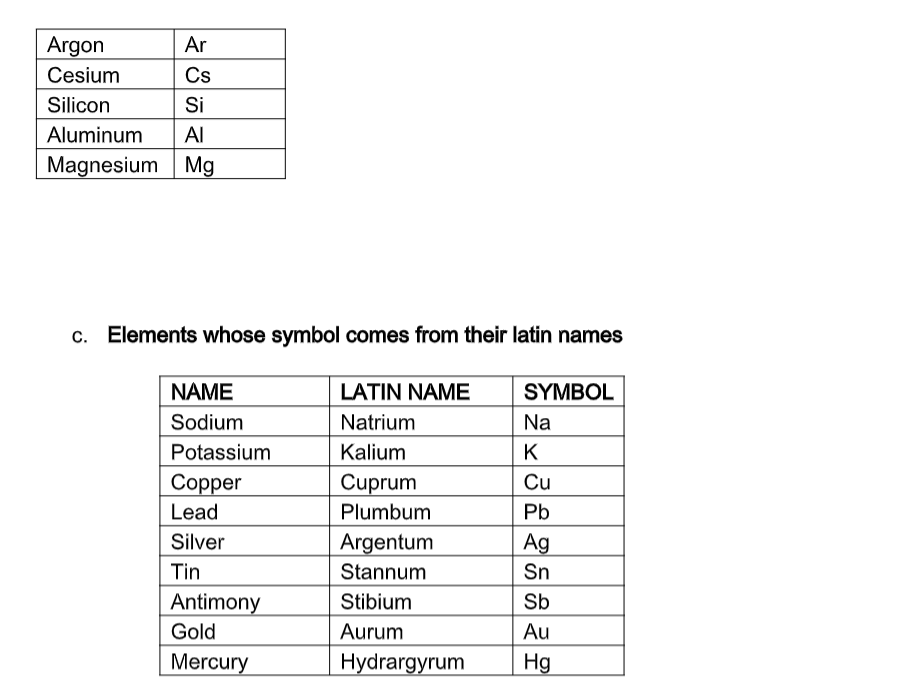
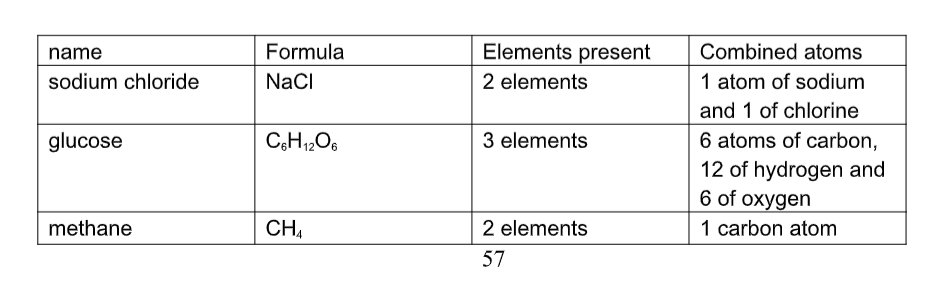

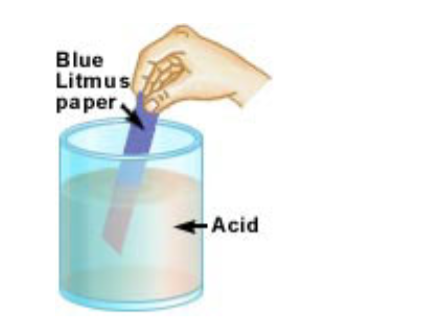
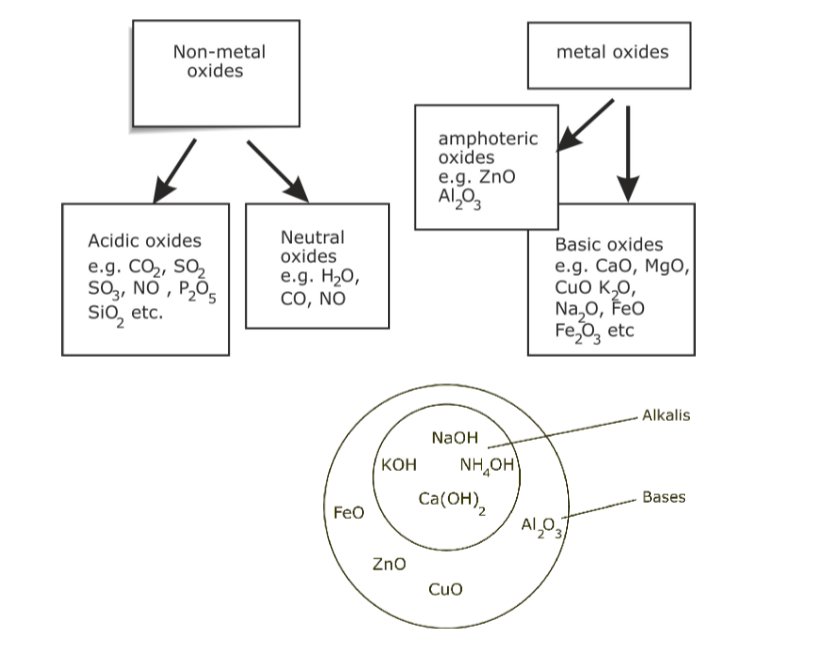
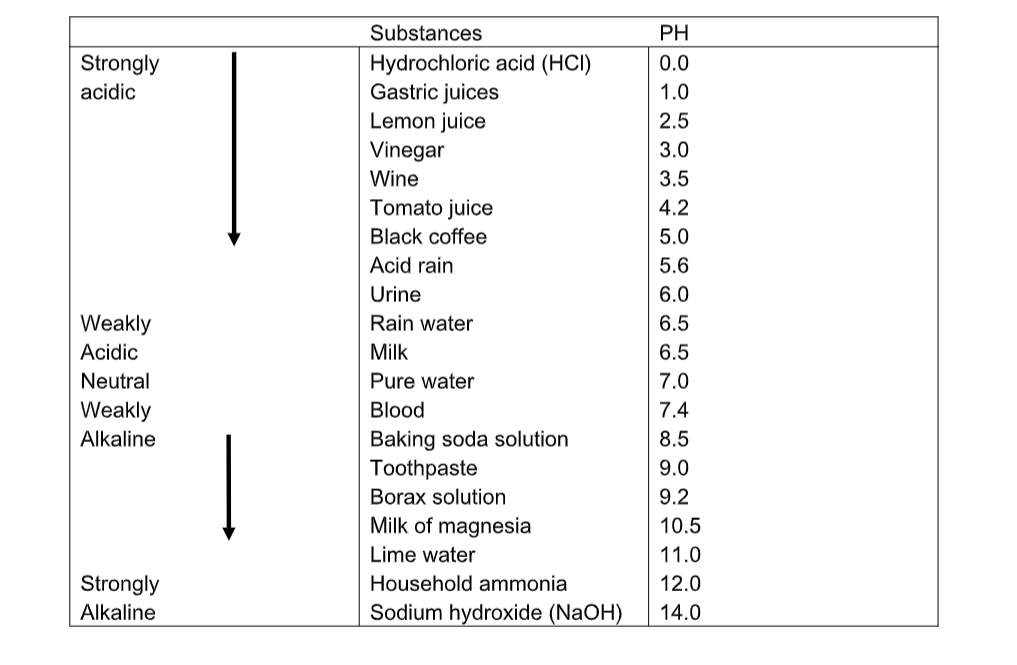
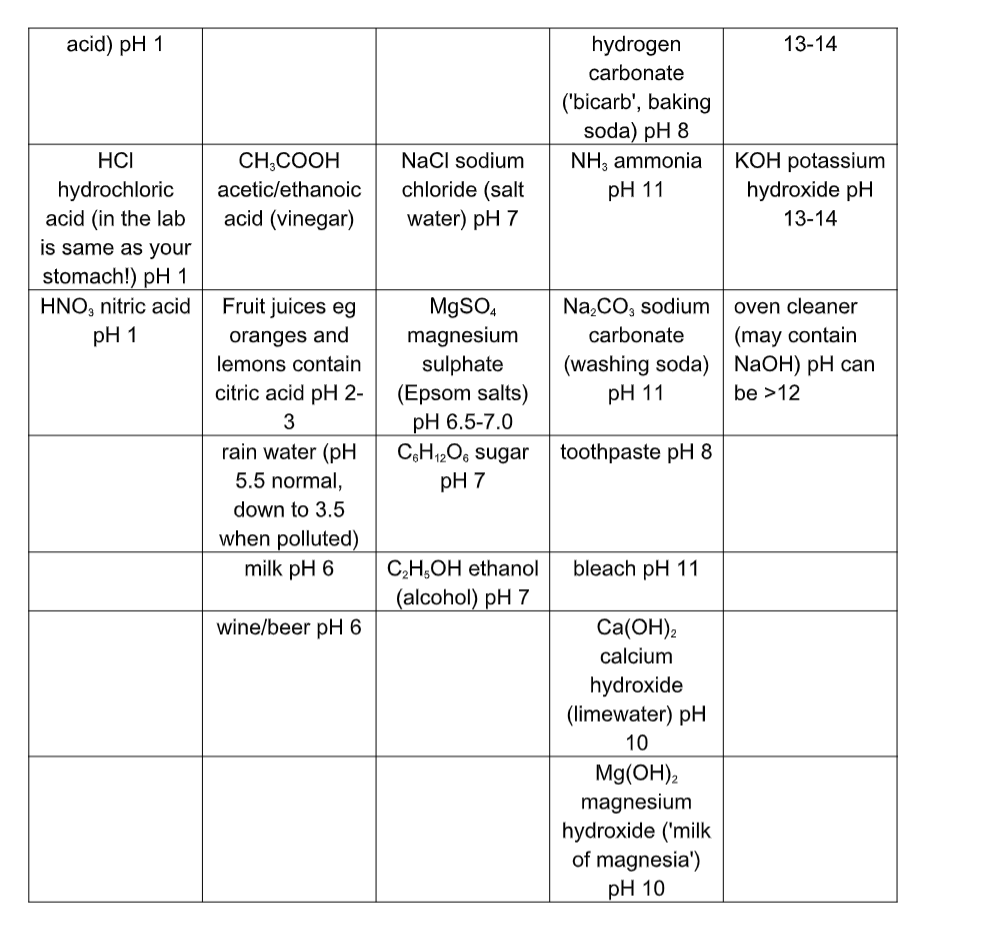
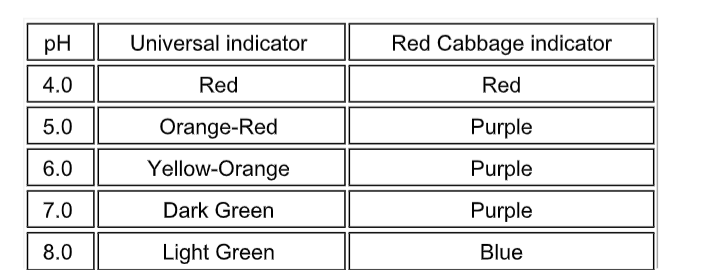
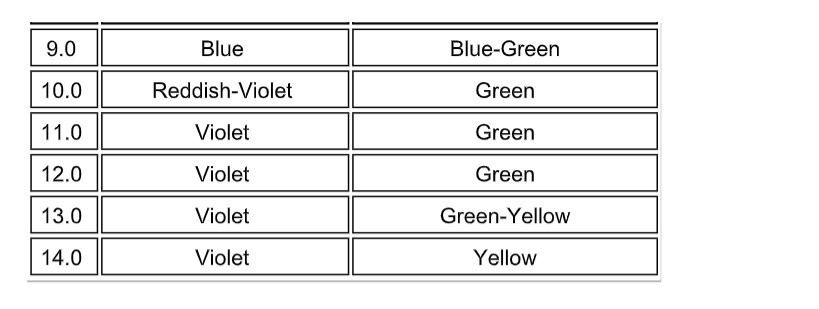
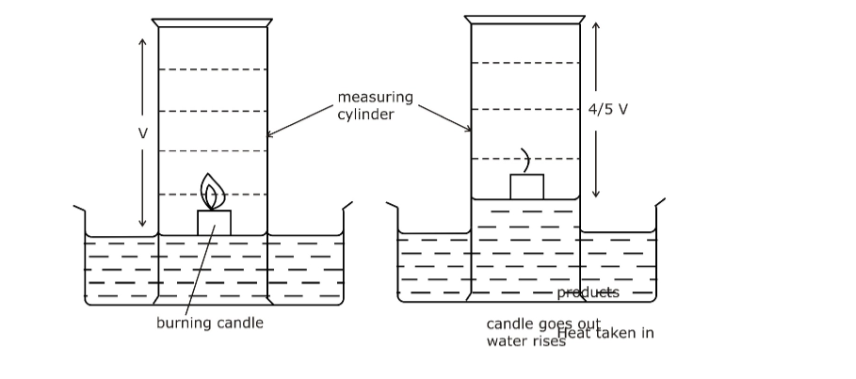
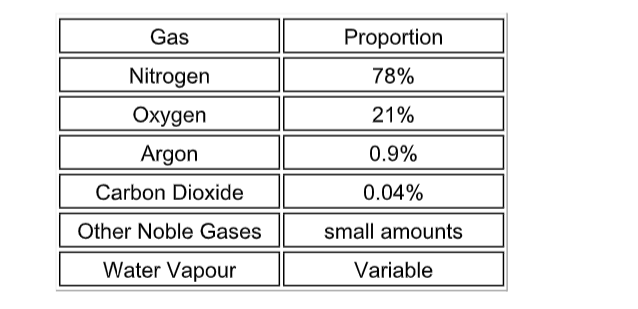
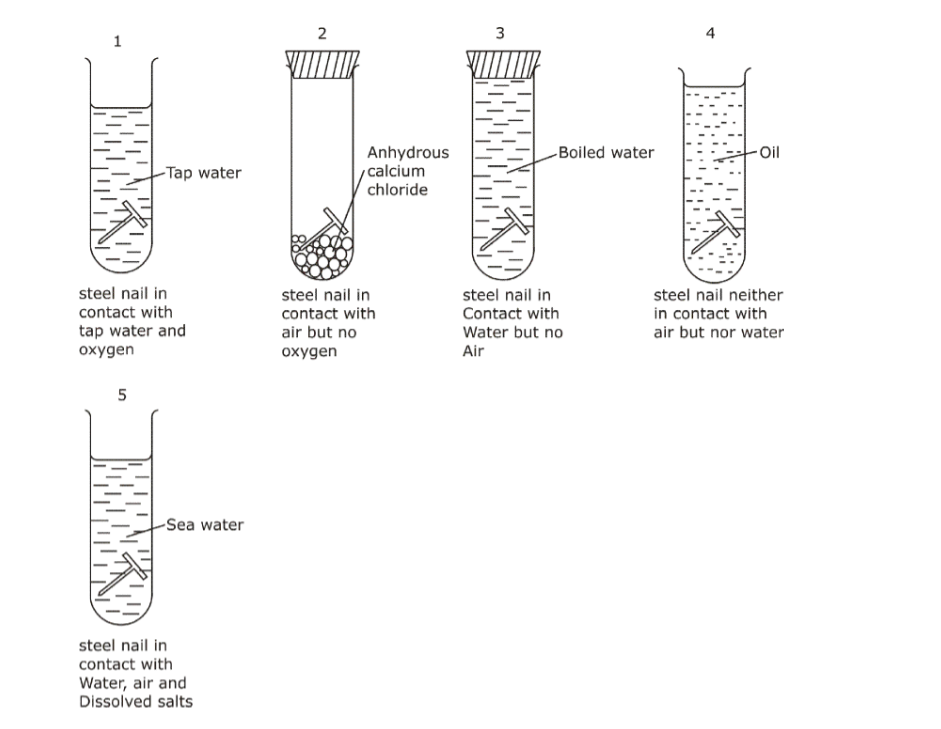
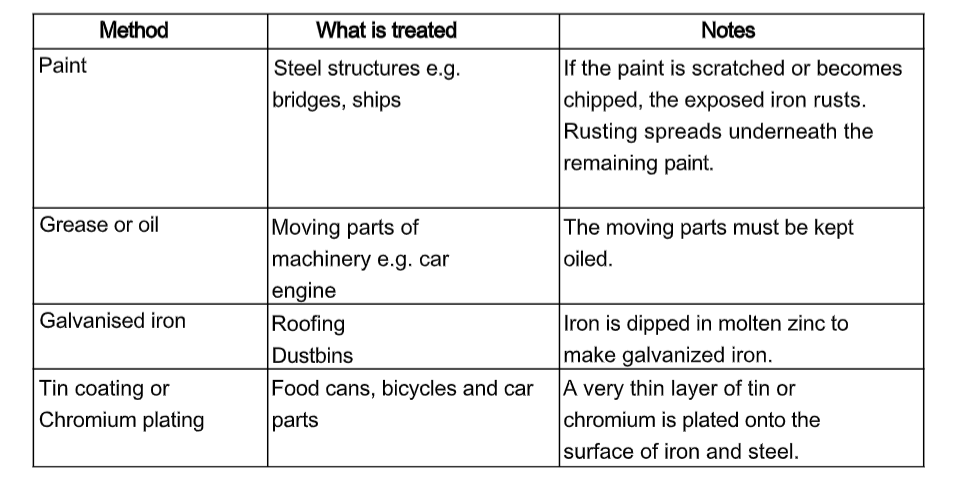
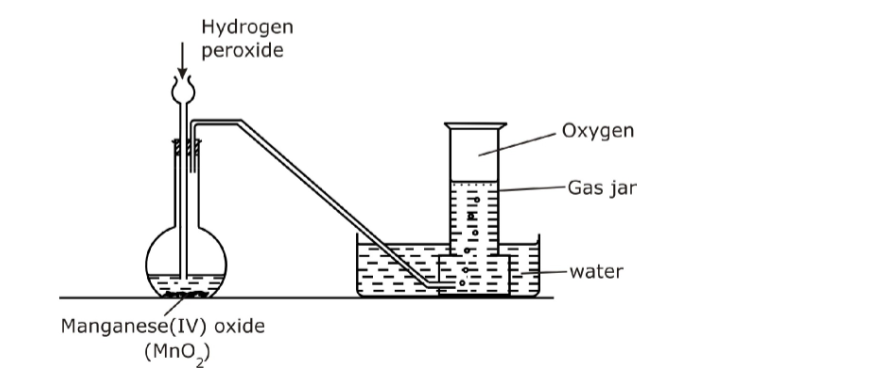
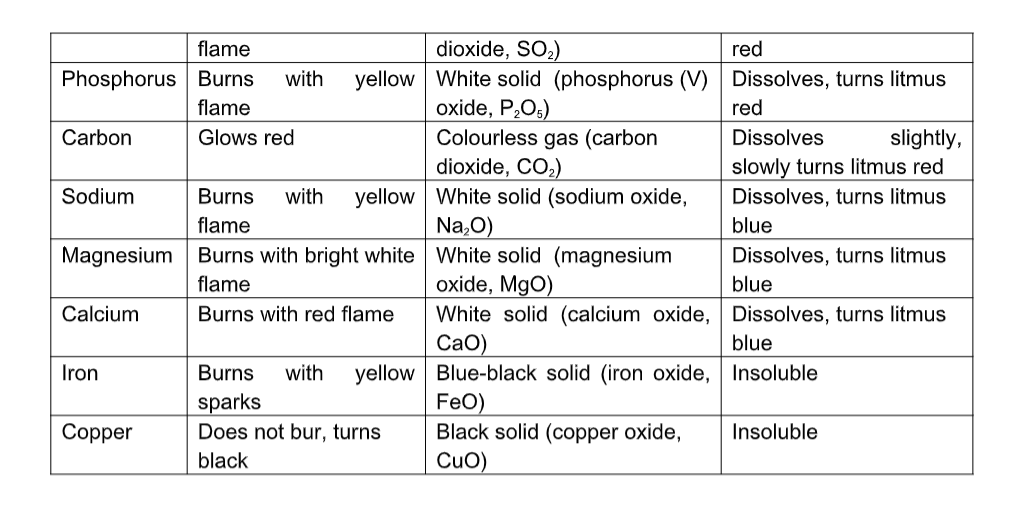
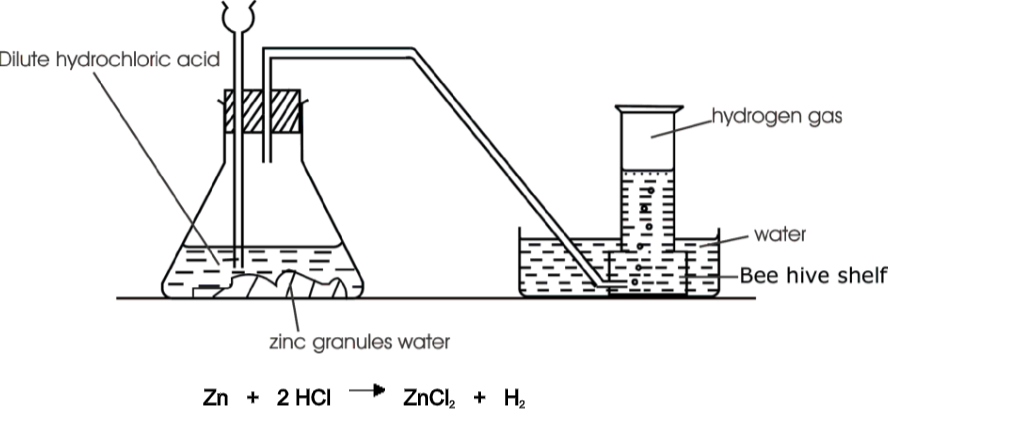
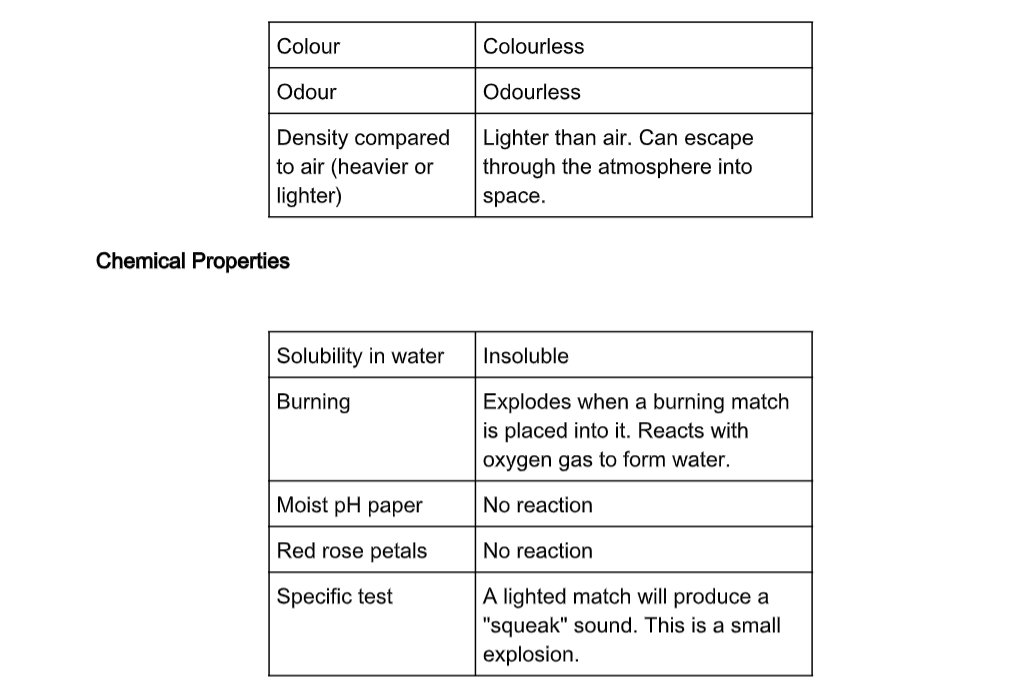
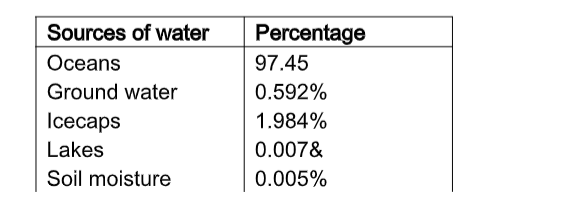

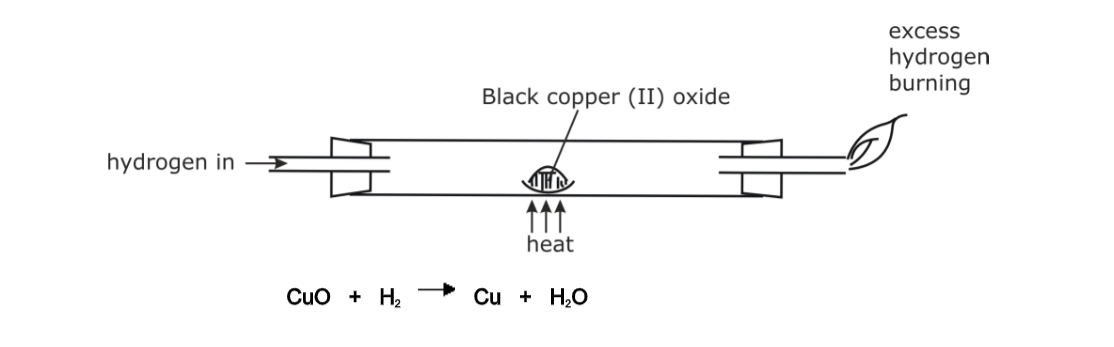
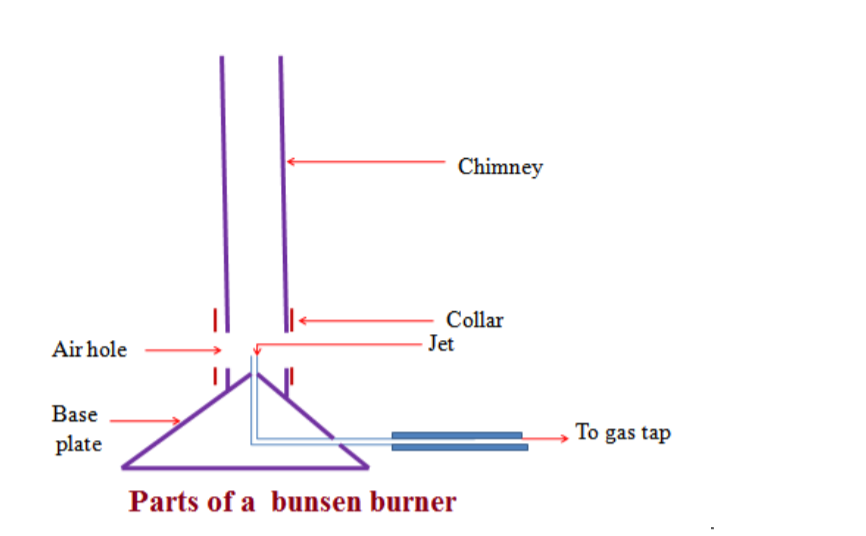
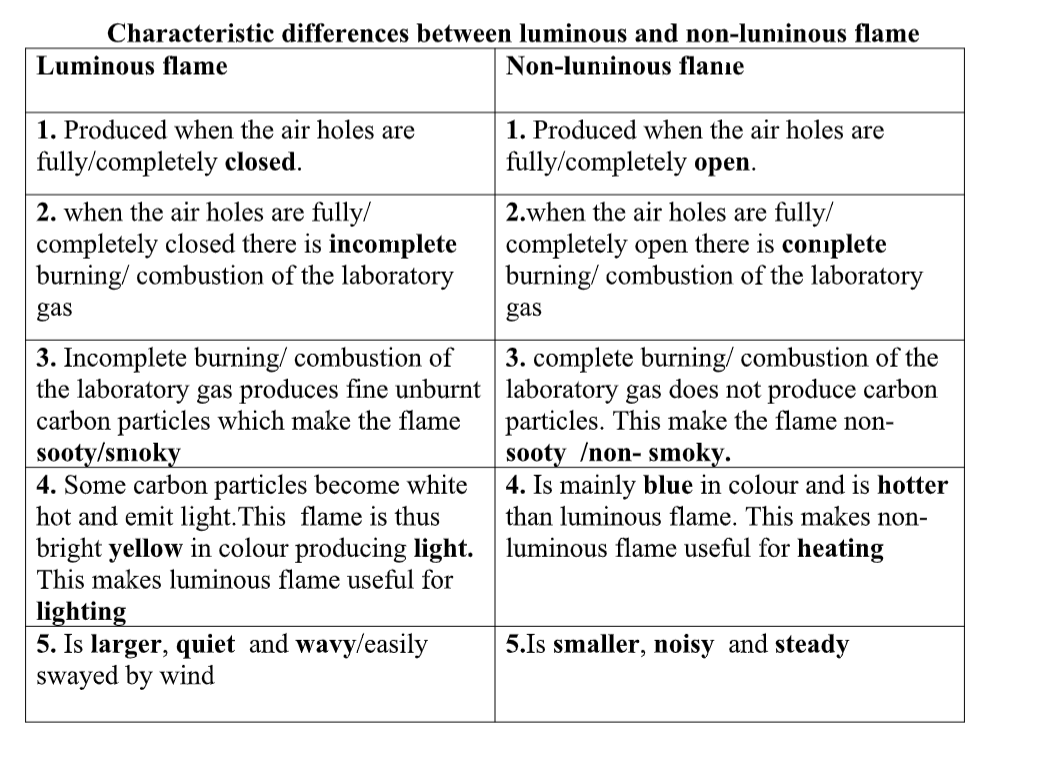
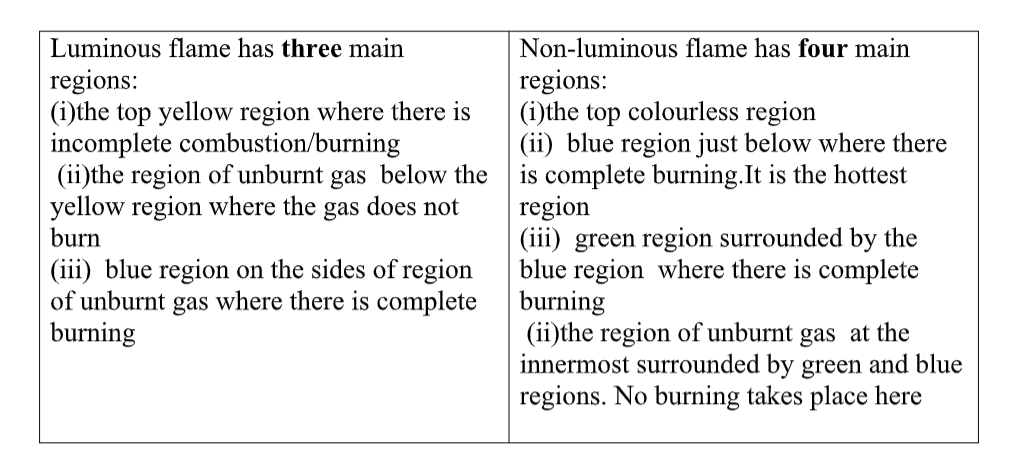
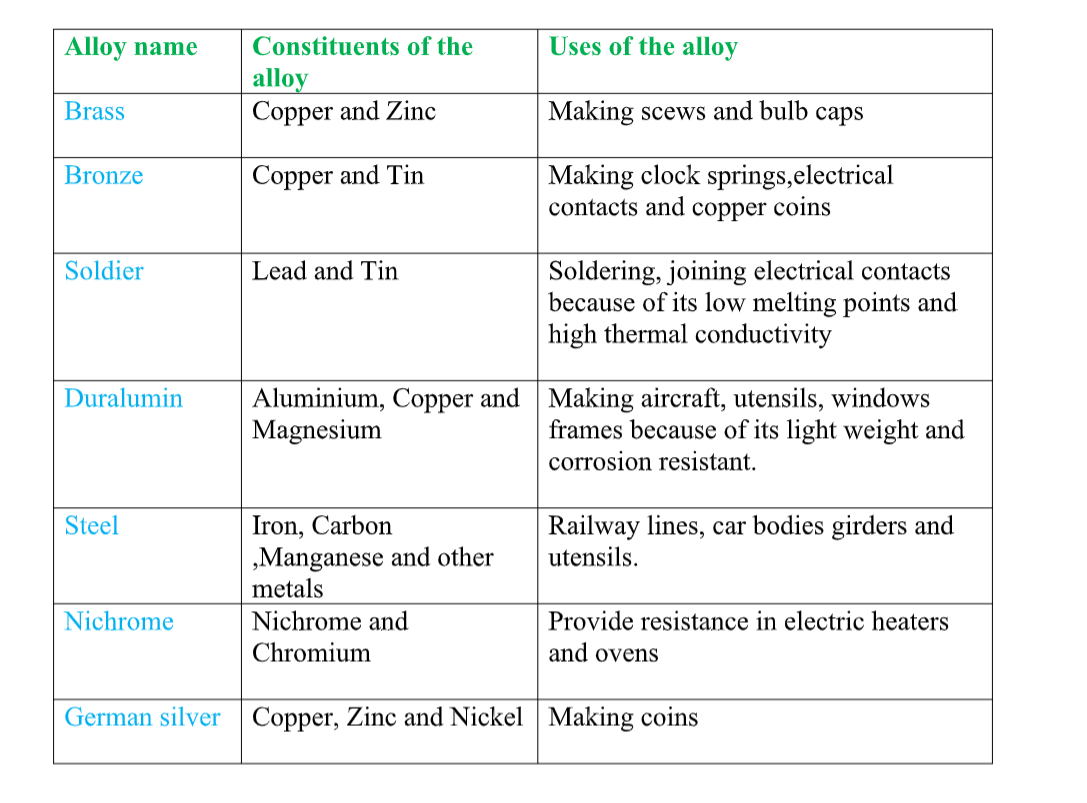
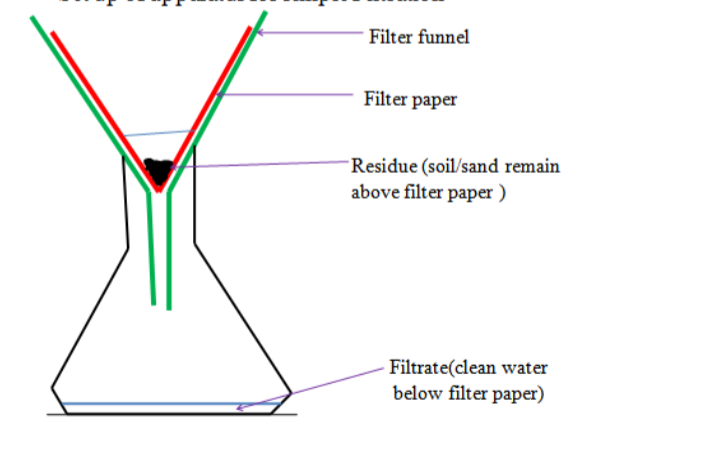
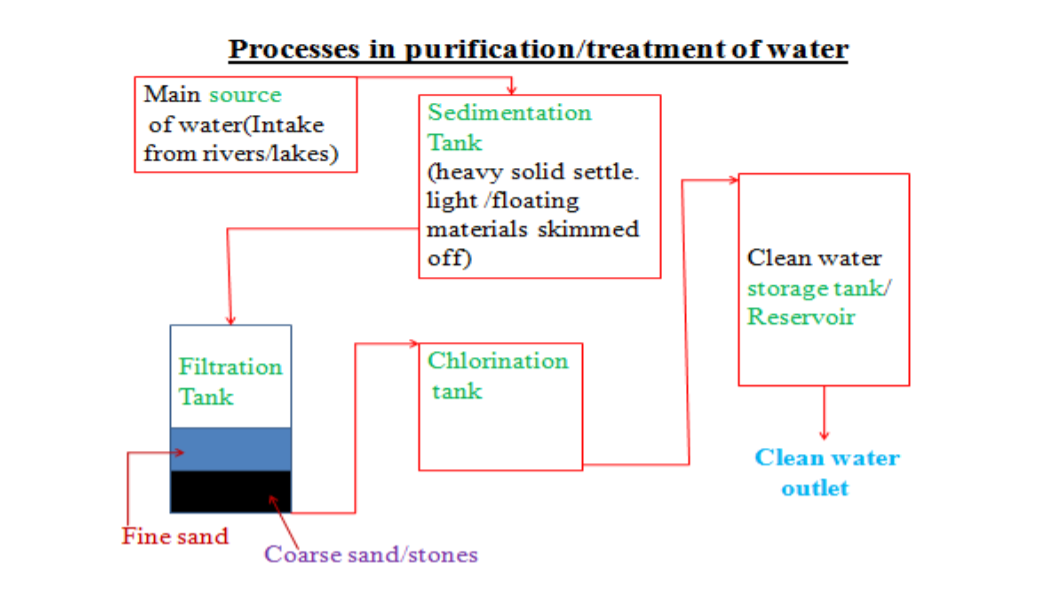
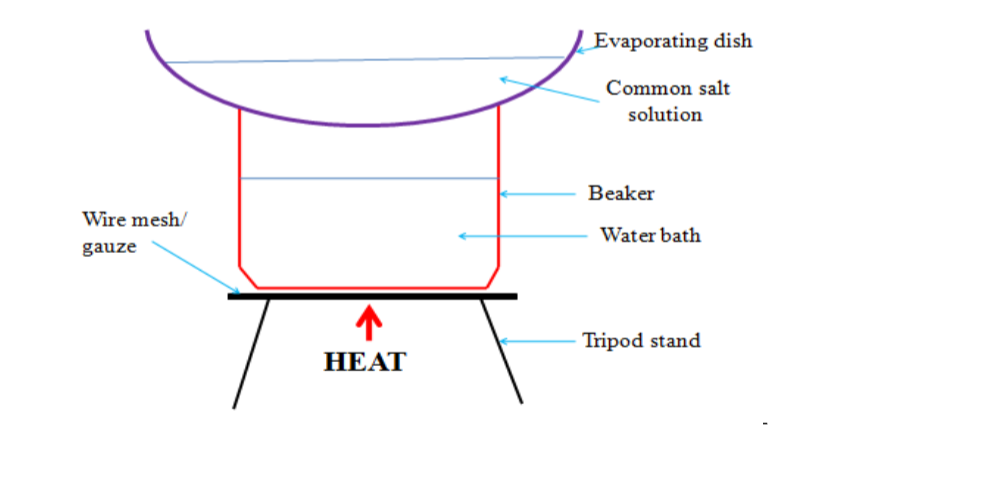
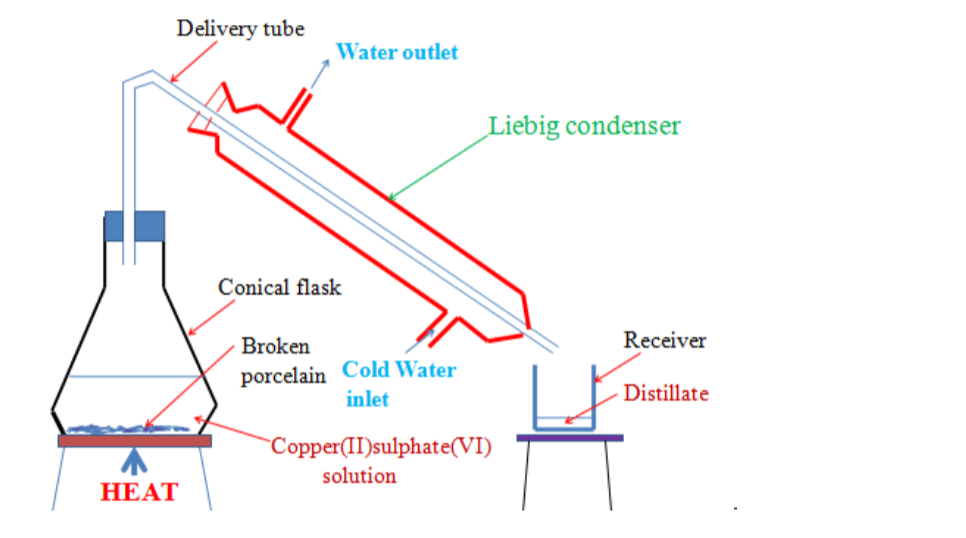
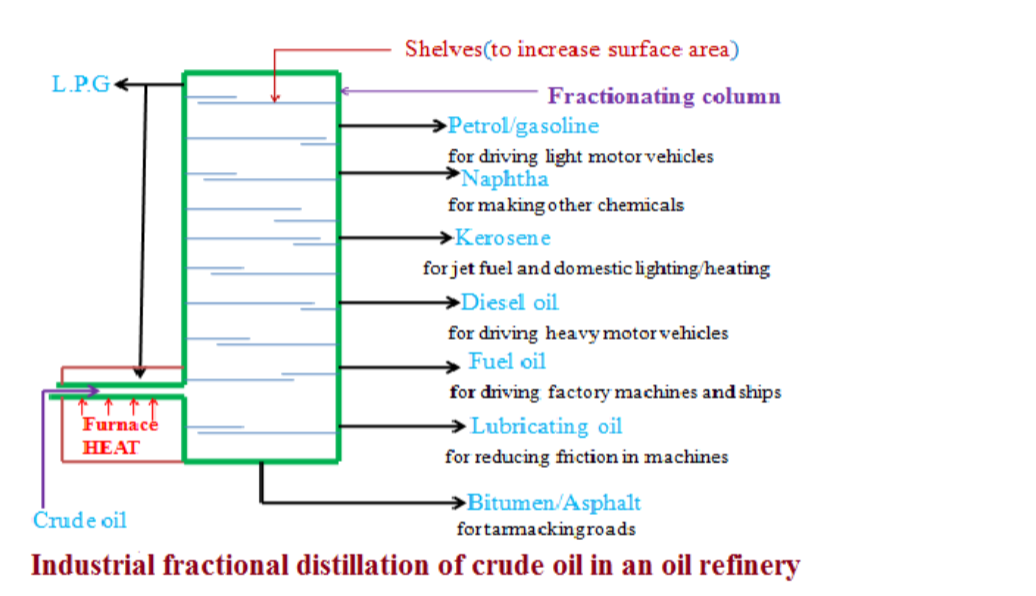
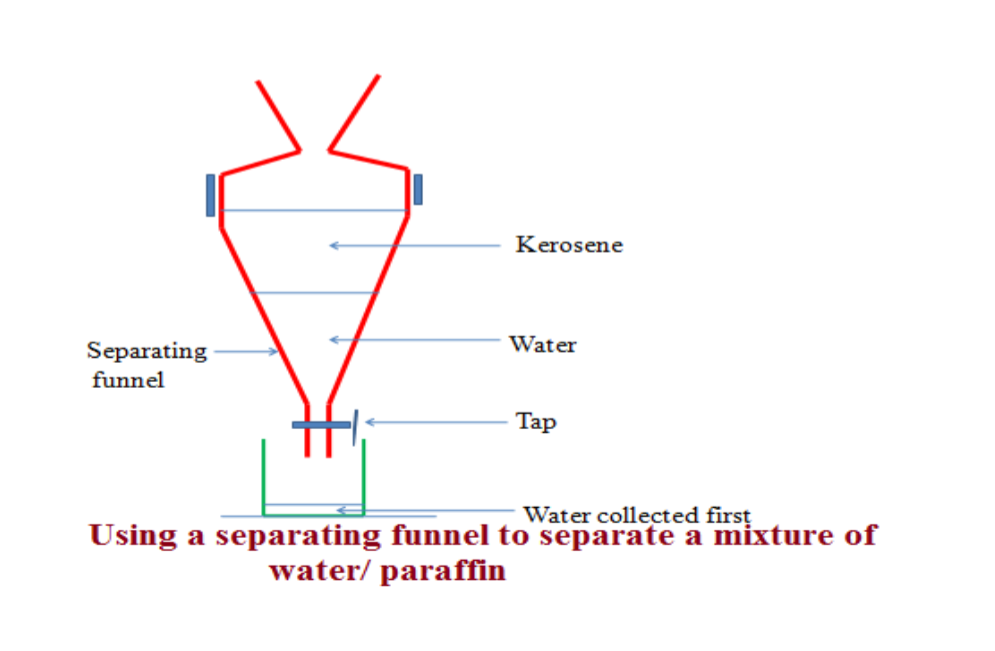
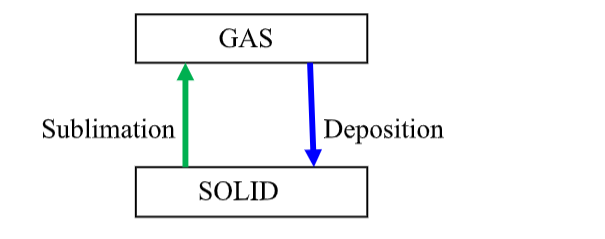
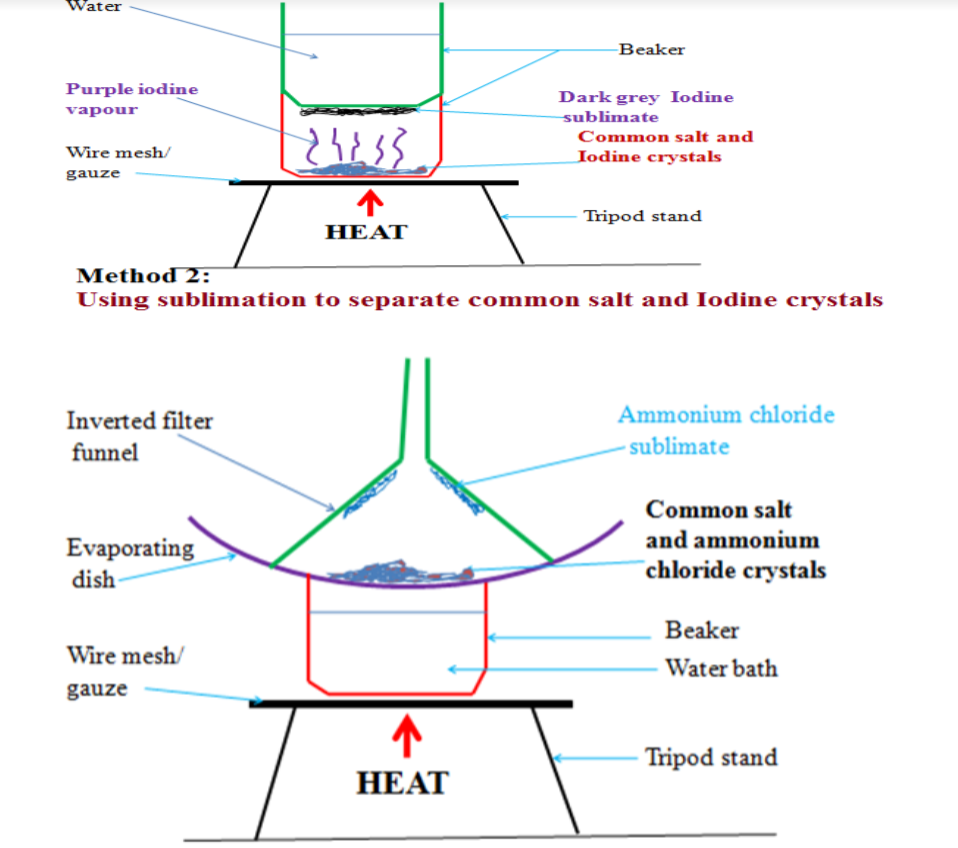
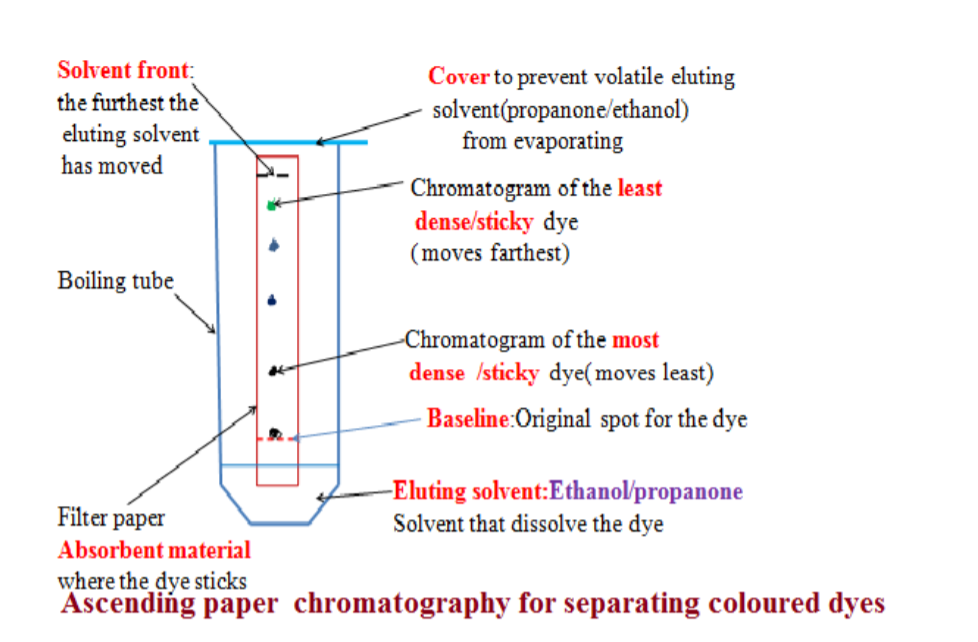
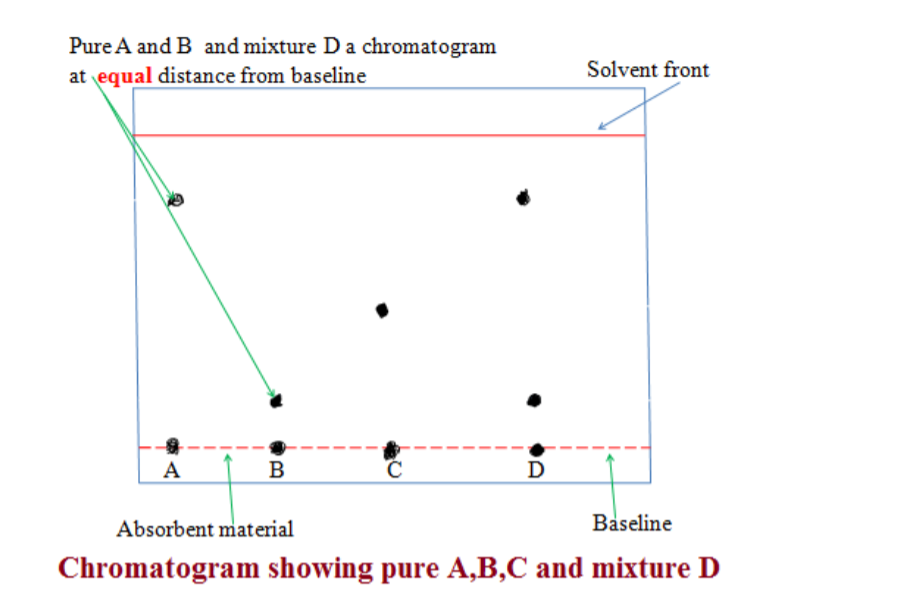
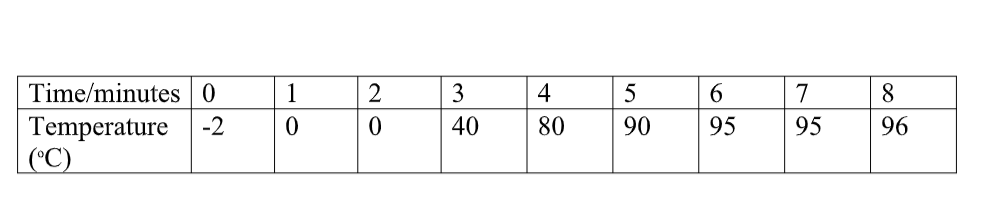
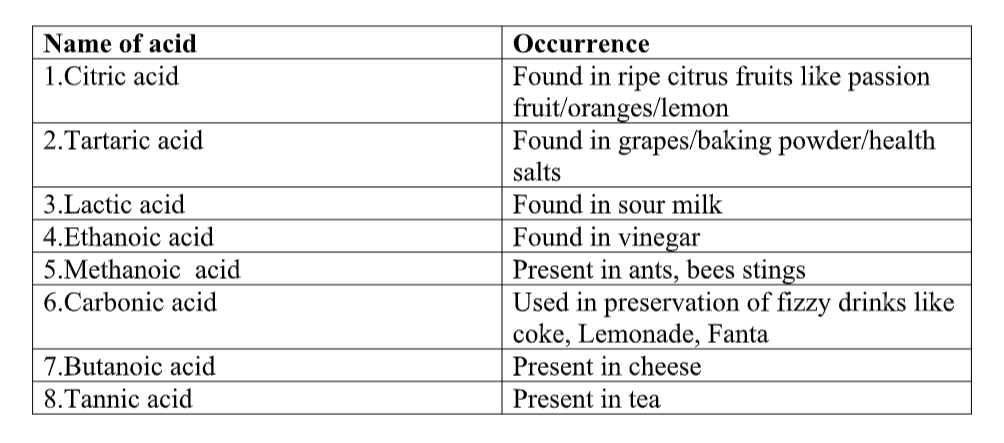

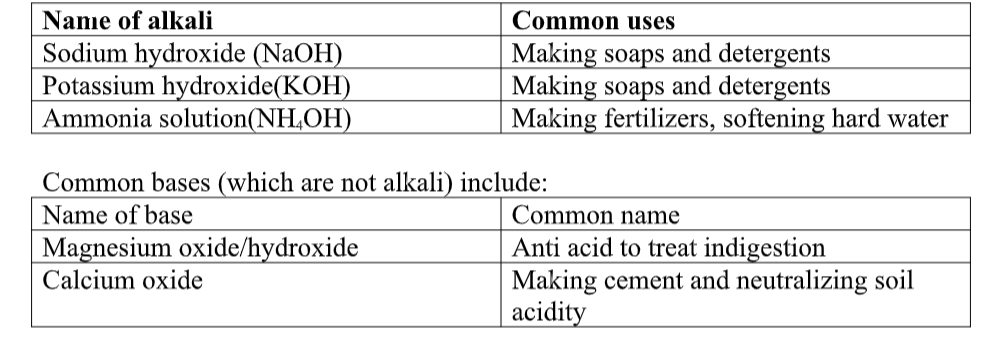
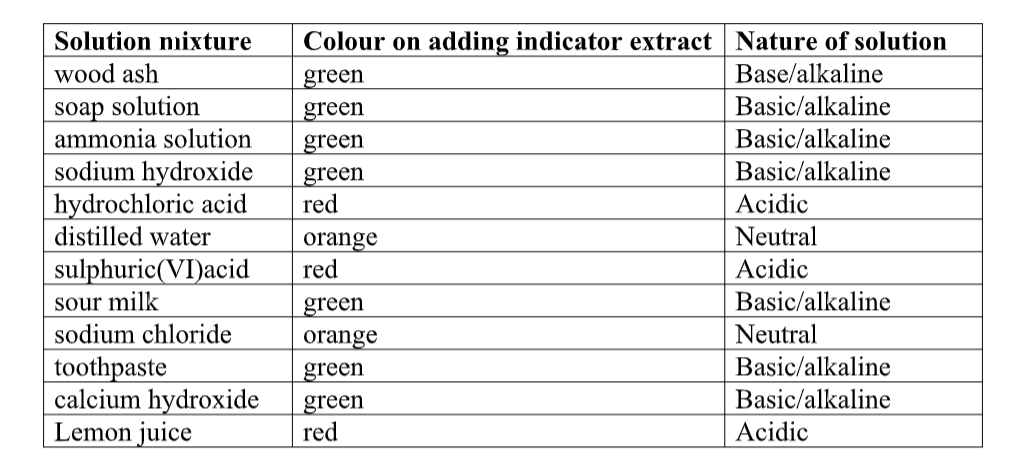
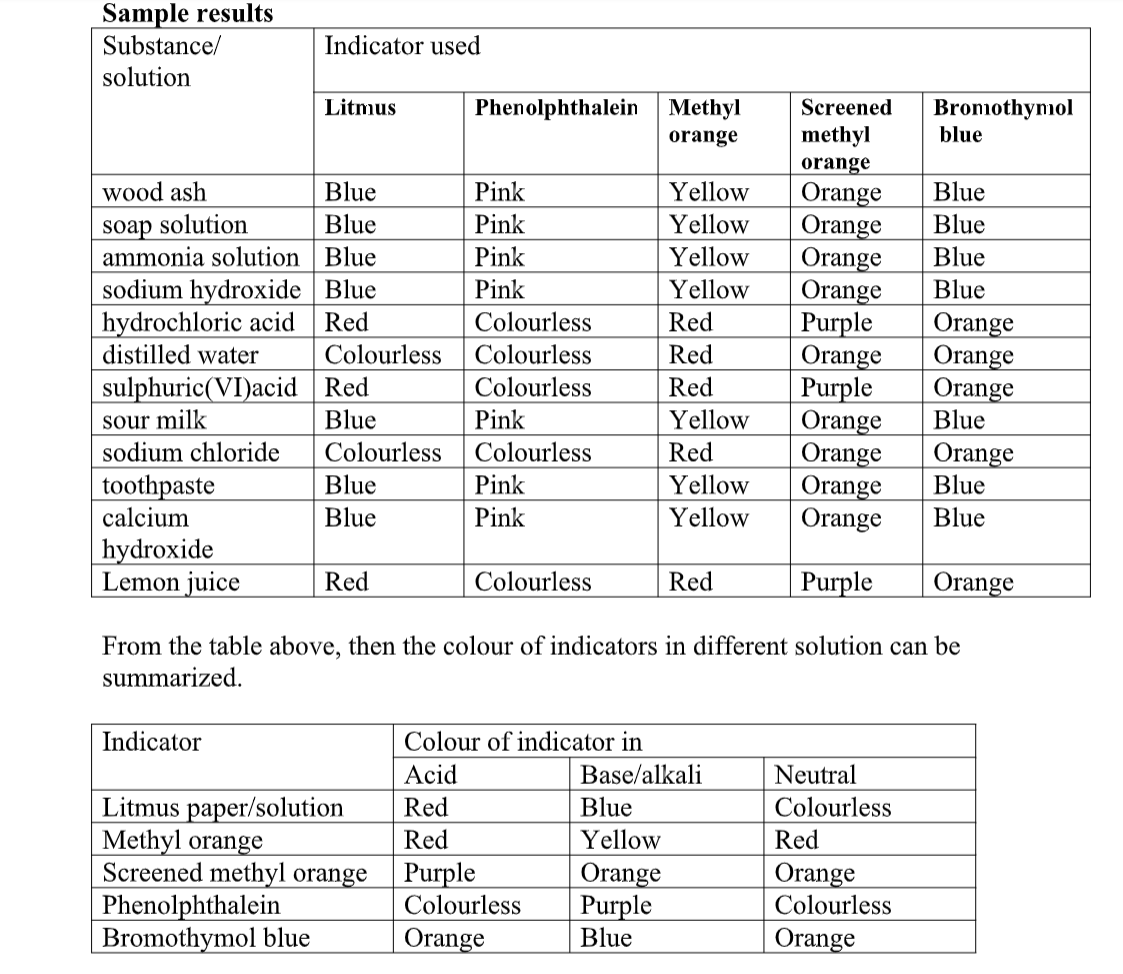
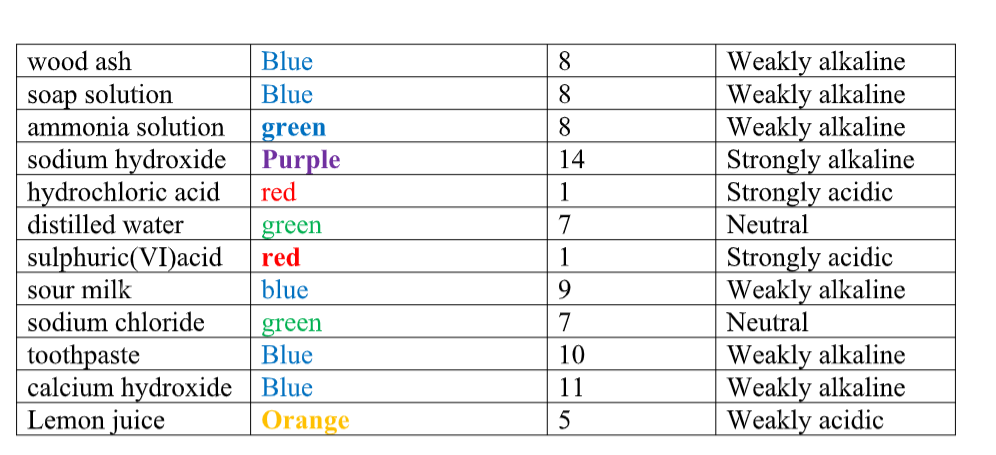
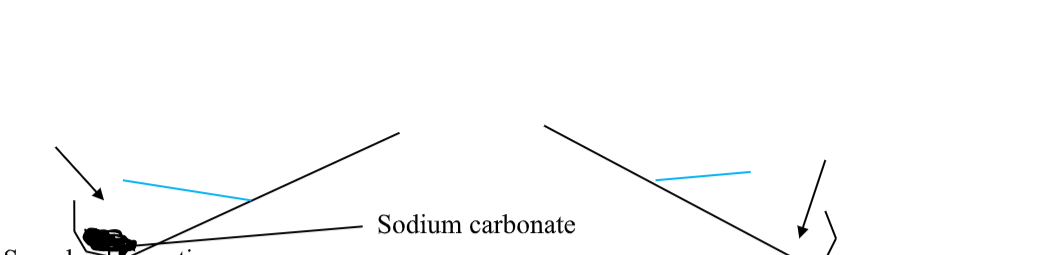
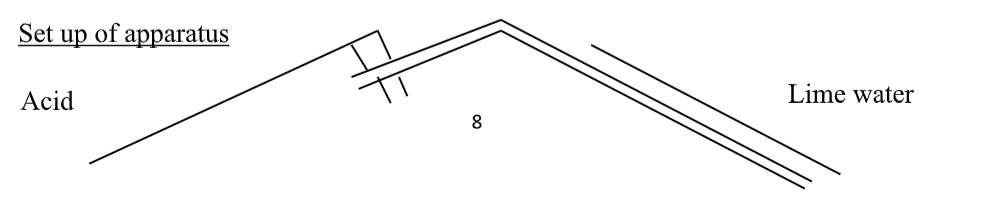
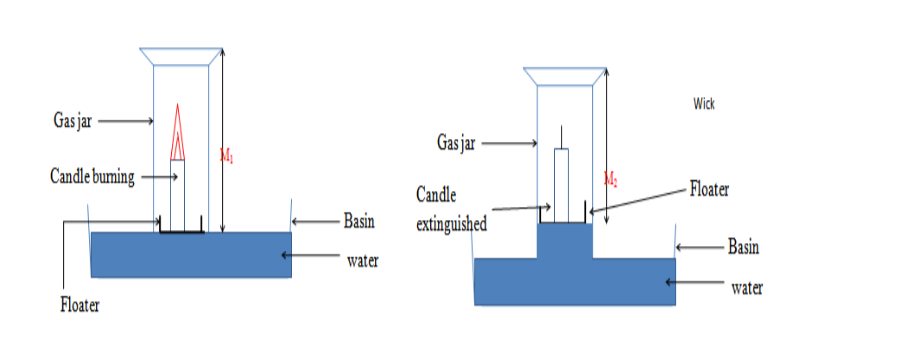
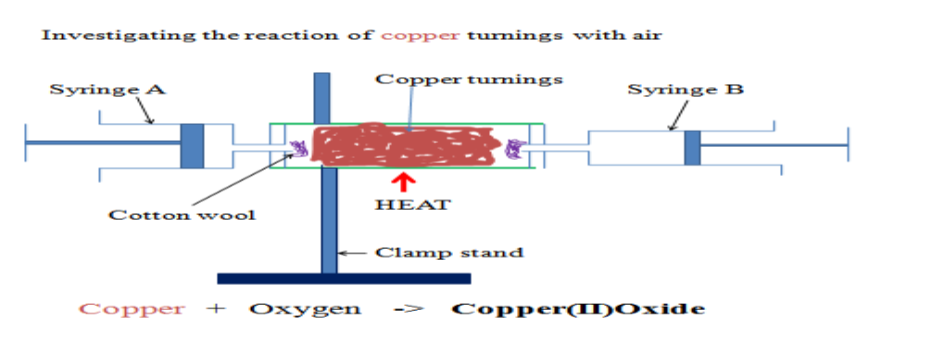
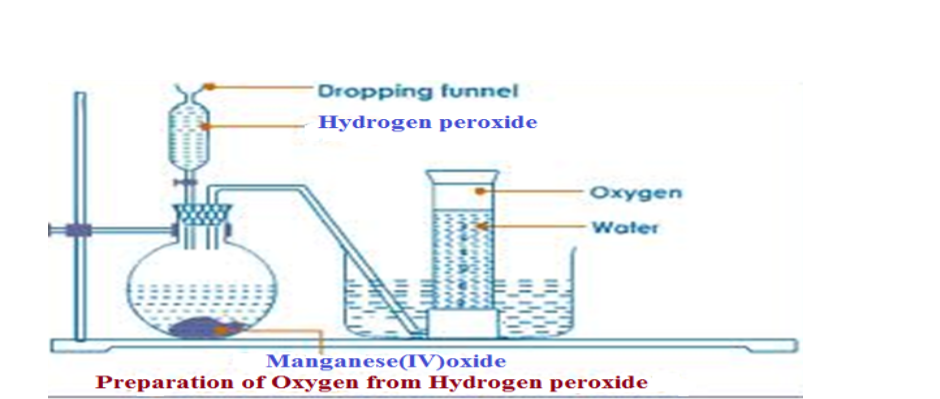
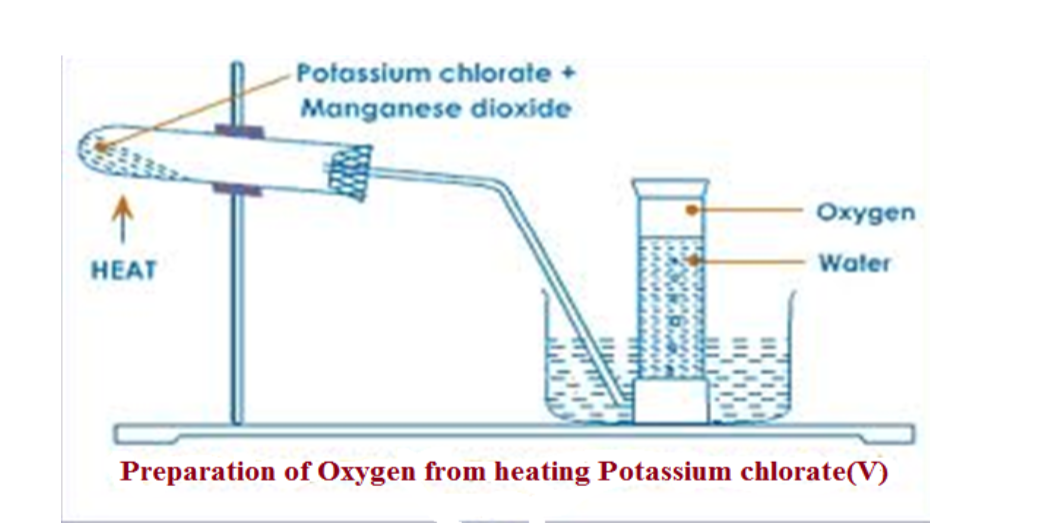

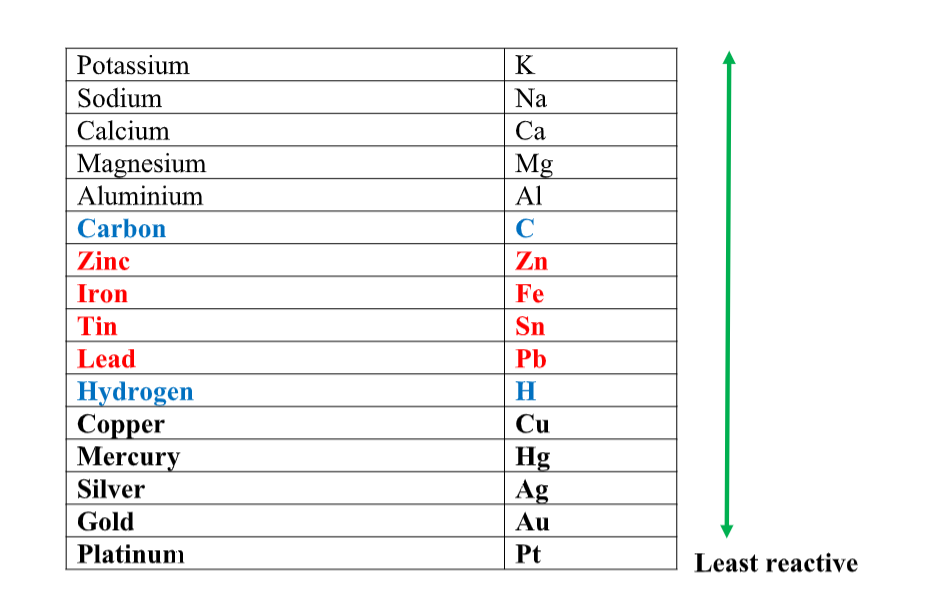
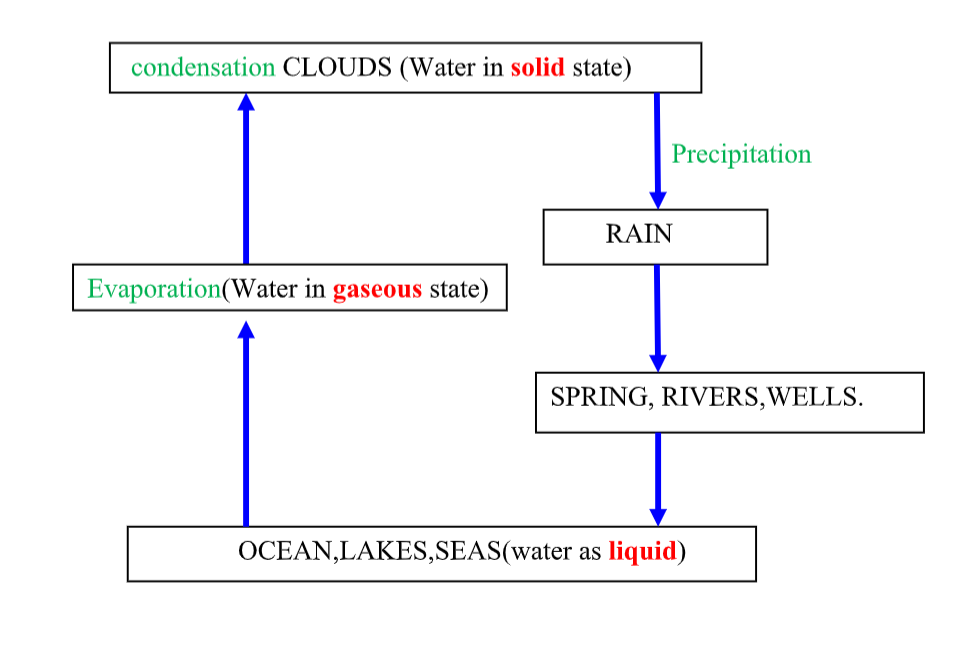
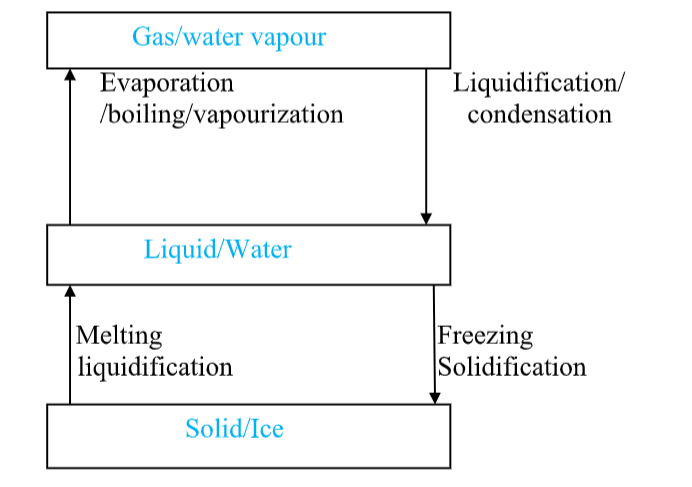
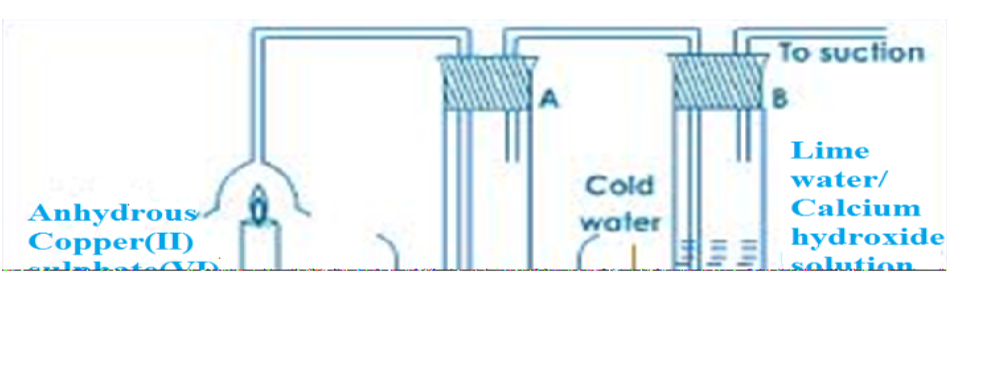
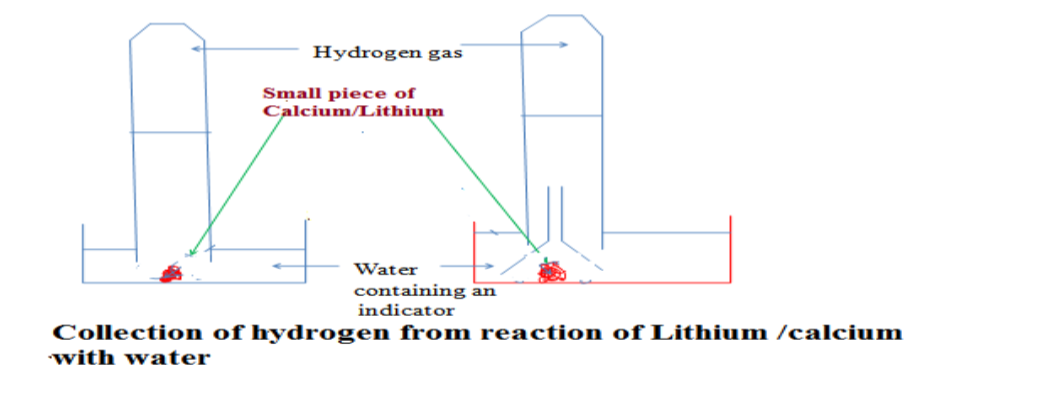
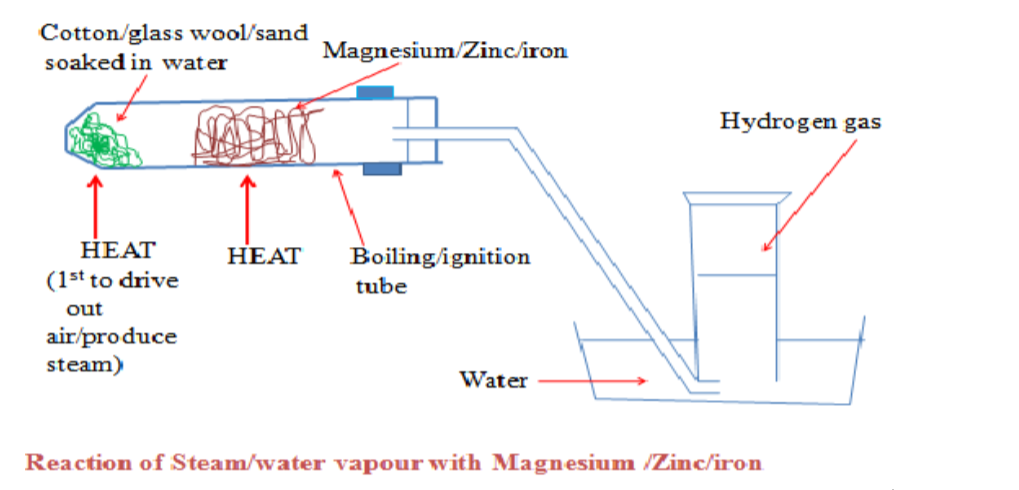
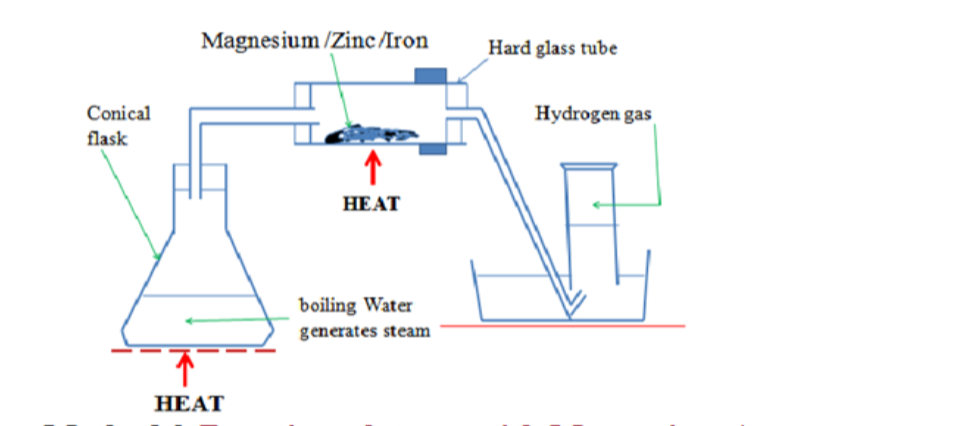
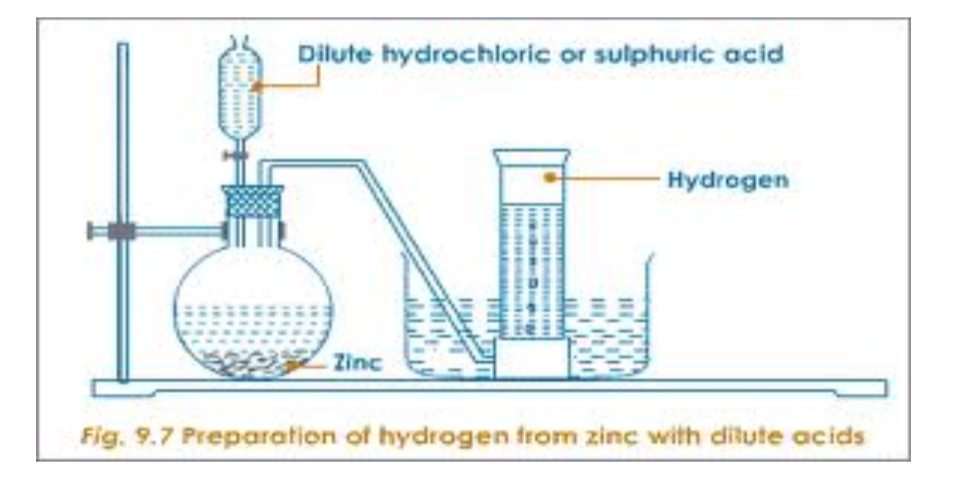
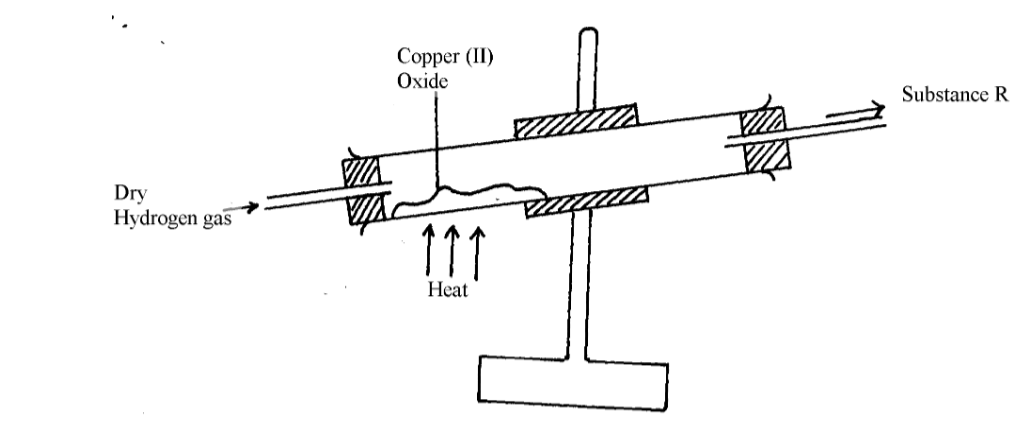
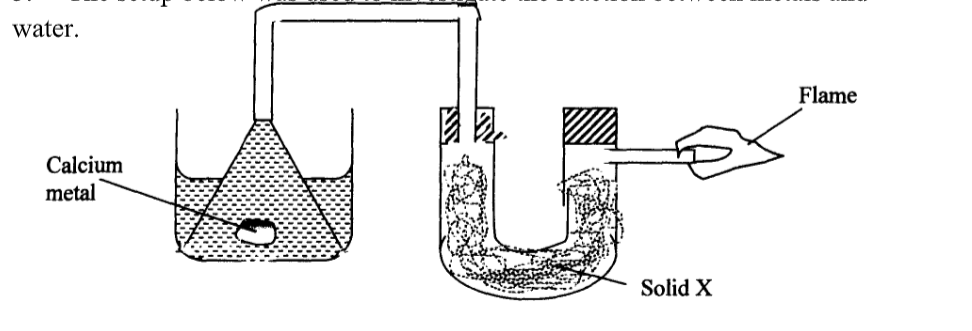
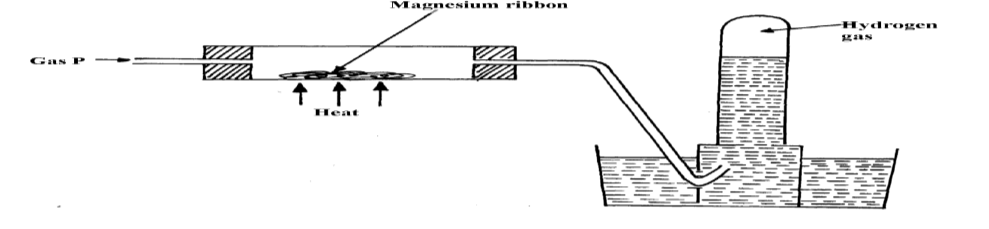
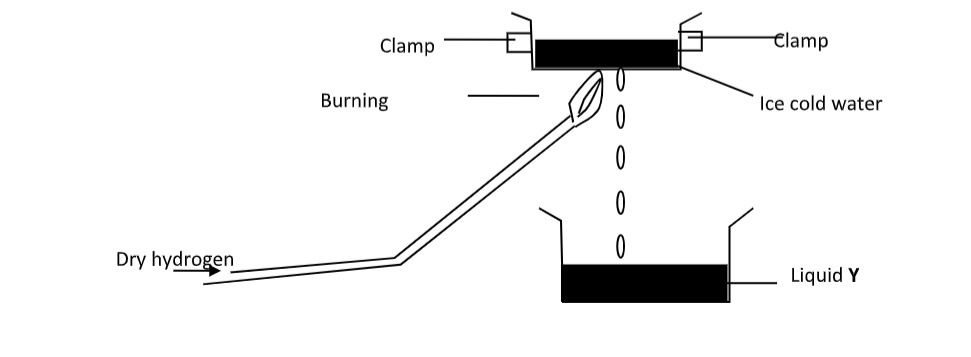
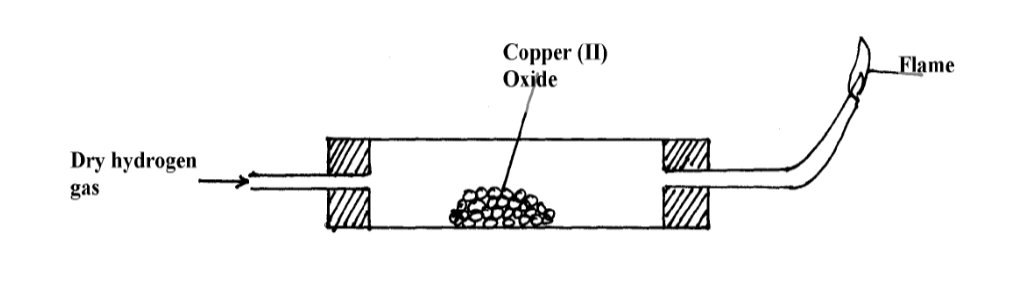
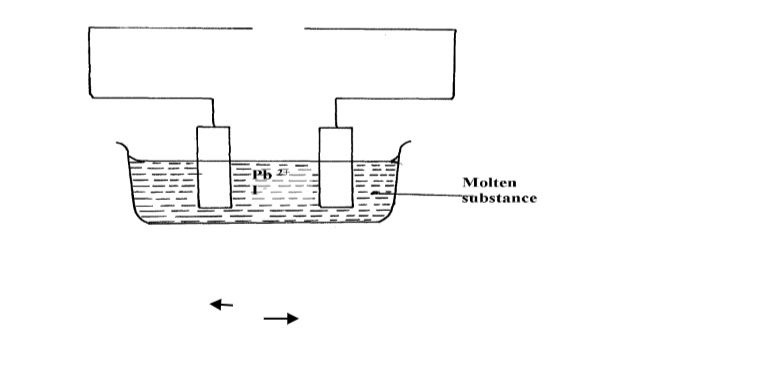
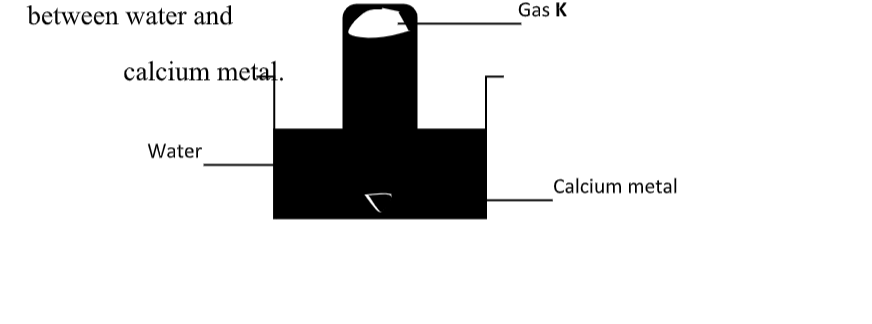
Leave a Reply